Main Menu (Mobile)- Block
- Overview
-
Support Teams
- Overview
- Anatomy and Histology
- Cryo-Electron Microscopy
- Electron Microscopy
- Flow Cytometry
- Gene Targeting and Transgenics
- High Performance Computing
- Immortalized Cell Line Culture
- Integrative Imaging
- Invertebrate Shared Resource
- Janelia Experimental Technology
- Mass Spectrometry
- Media Prep
- Molecular Genomics
- Stem Cell & Primary Culture
- Project Pipeline Support
- Project Technical Resources
- Quantitative Genomics
- Scientific Computing
- Viral Tools
- Vivarium
- Open Science
- You + Janelia
- About Us
Main Menu - Block
- Overview
- Anatomy and Histology
- Cryo-Electron Microscopy
- Electron Microscopy
- Flow Cytometry
- Gene Targeting and Transgenics
- High Performance Computing
- Immortalized Cell Line Culture
- Integrative Imaging
- Invertebrate Shared Resource
- Janelia Experimental Technology
- Mass Spectrometry
- Media Prep
- Molecular Genomics
- Stem Cell & Primary Culture
- Project Pipeline Support
- Project Technical Resources
- Quantitative Genomics
- Scientific Computing
- Viral Tools
- Vivarium
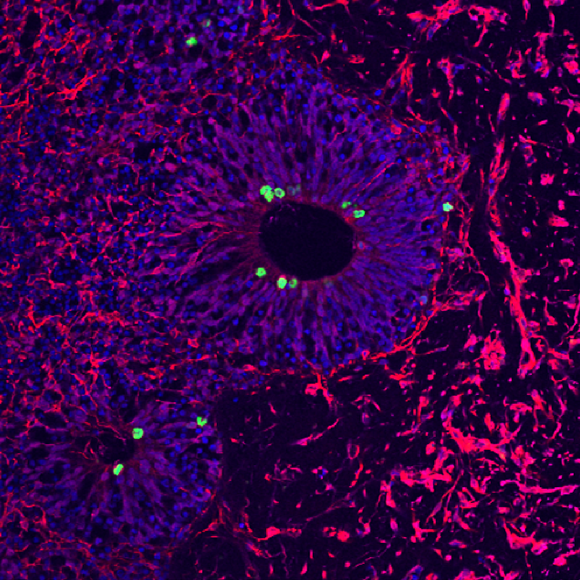
Our lab is focused on investigating interactions between neurons and glia in normal physiology and in disease.
Our Research
Mechanisms of synapse elimination
We are broadly interested in how interactions between glia and neurons alter neuronal function in normal and diseased conditions. Our ongoing project aims to understand how glial cells specifically eliminate excessive and weak synapses to form mature neuronal circuits during a postnatal developmental process known as synapse elimination or synapse refinement.
We aim to understand which molecules and signaling pathways link weak synapse activity to synapse elimination, as well as the mechanisms through which the phagocytic machinery of astrocytes and microglia recognizes and removes such synapses.
Role of mitochondria in regulation of dendrite Ca2+ dynamics
Calcium signaling plays a critical role in relaying specific patterns of synaptic activation to the cell body. Nevertheless, how calcium signals are integrated over multiple synapses and transmitted to the cell body is not fully understood. We are interested in whether intracellular calcium reservoirs such as mitochondria and endoplasmic reticulum, tune cytosolic calcium dynamics to ensure coherent signals are propagated from dendrites to axons. To study this question, we combine electrophysiology with calcium imaging techniques to simultaneously track calcium dynamics and electrophysiological readouts in response to the perturbation of mitochondrial calcium regulation.
Neurotransmitter receptor trafficking
We are also interested in how neurons traffic neurotransmitter receptors to and from specific synapses undergoing plasticity. We use CRISPR/Cas9 techniques to tag receptors that are expressed from their native loci, and single particle tracking to characterize the motion of receptors as they travel in vesicles through dendrites. In addition, we examine how the rearrangement of cytoskeletal elements during neuronal activity modulates the motion of receptors.
