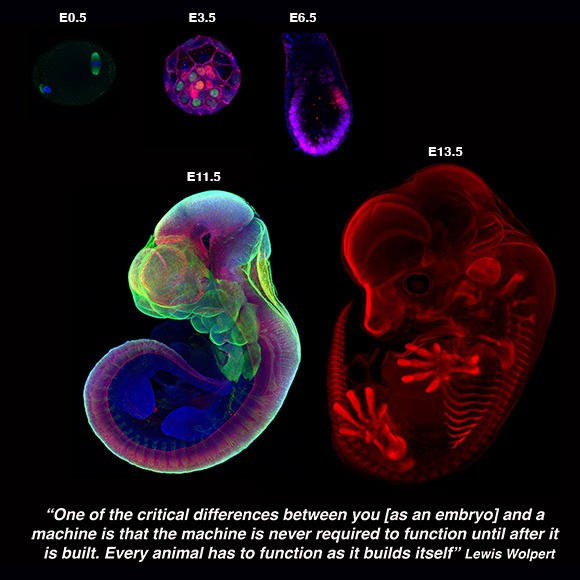
We are interested in reconstituting mammalian development by devising ex utero culture systems for natural embryos and stem-cell derived embryo models. We aim to harness these platforms to understand how cells build embryos and how embryos build tissues and organs.
Reconstituting mammalian embryogenesis ex utero
Every embryo begins as a simple sphere of cells, with no front or back, head or tail. In placental mammals (including humans), patterning of the future body of the embryo begins soon after it implants into the maternal uterus, during one of the most important and poorly understood phases of embryogenesis: gastrulation. During this period, the pluripotent epiblast differentiates into the three major germ-layers (endoderm, ectoderm, and mesoderm), and the embryo transits from a symmetrical ball of stem cells into an animal with a defined body plan and embryonic axis, establishing the internal organization as well as the external form of the developing embryo, to subsequently commence organ formation.
Studying the mechanisms governing mammalian embryo development during these stages is crucial for understanding the basis of developmental defects, pregnancy loss, and for learning the developmental principles that can be applied in differentiation of stem cells for cell and organ replacement therapies. Yet, the small size and intrauterine confinement of the developing mammalian embryo, together with the inability to transfer embryos back to the uterus at any stage after implantation, limits our experimental capacity to observe and manipulate gastrulating embryos, restricting the study of early post-implantation embryogenesis to developmental snapshots, which do not allow to follow the live progress of embryonic morphogenesis.
The ability to remove a mammalian embryo from the uterine environment and grow it normally under controlled conditions constitutes an exceptional tool for characterizing the effects of perturbations during development, allowing experimentation in living embryos during stages that remained inaccessible inside the maternal uterus.
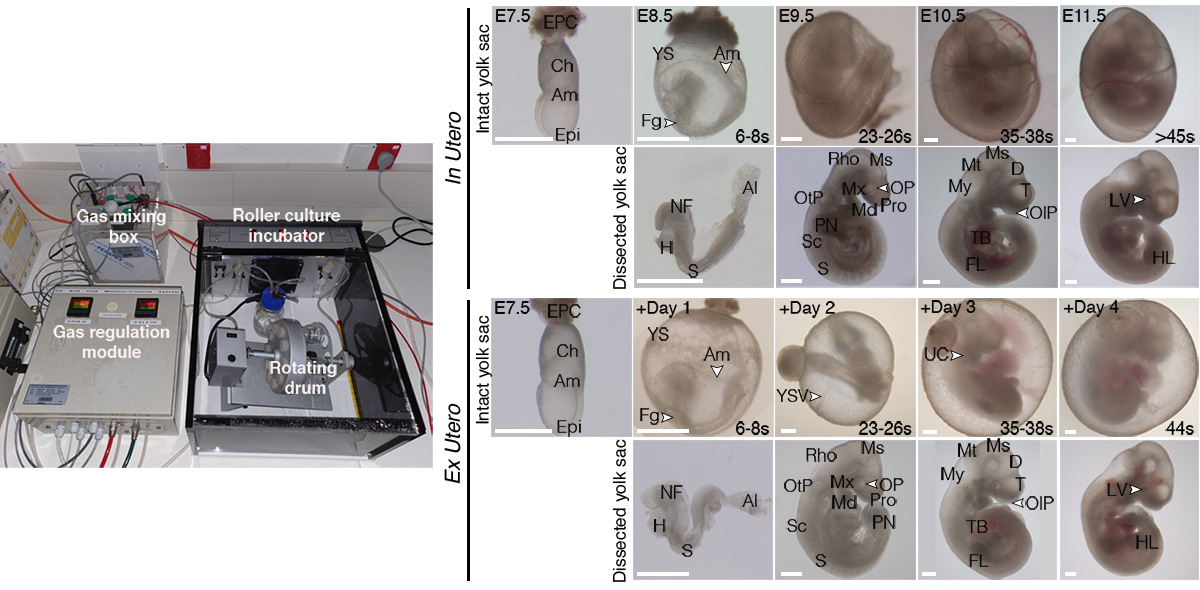
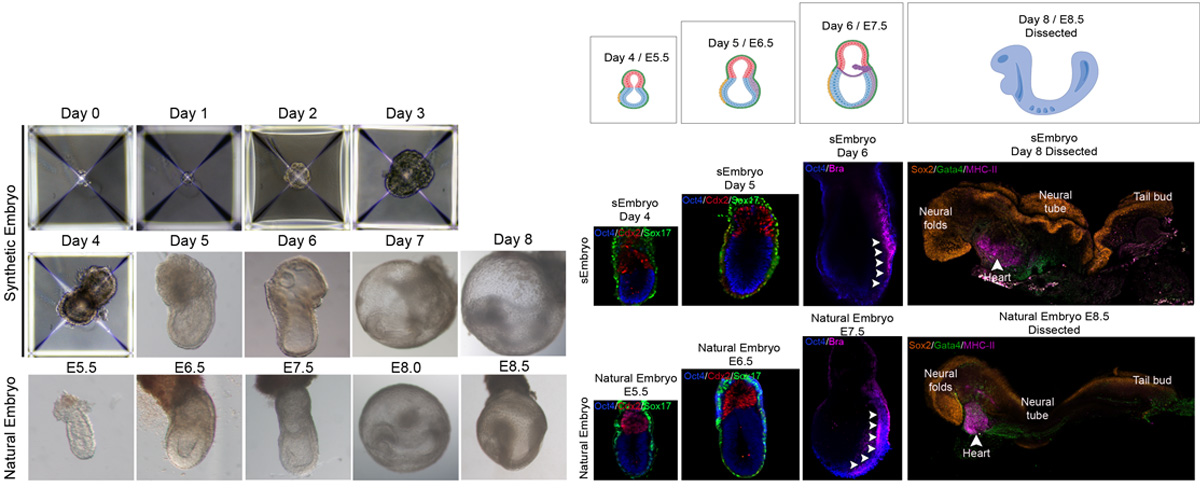
Ex utero embryogenesis of stem cell-derived embryo models
Recently, numerous studies have demonstrated the ability of embryonic stem cells (ESCs) to self-organize into embryo-like entities under specific in vitro culture conditions, providing an unprecedent opportunity to model specific windows of embryogenesis. The lack of effective platforms for capturing full gastrulation of natural embryos ex utero, in addition to the impossibility of transferring post-implanted embryos back into the uterus of a surrogate mother made it unfeasible to test the developmental potential of these embryo models. By further adapting the aforementioned ex utero culture conditions, we demonstrated that mouse stem cell-embryo models (SEMs or sEmbryos) assembled solely from naïve ESCs are able to recapitulate the full process of gastrulation until early organogenesis adequately in vitro. These stem-cell-derived embryos include both embryonic and extraembryonic compartments, and recapitulate spatial and temporal events of early natural mouse embryogenesis from E5.5 to E8.5, such as cavitation, symmetry breaking, gastrulation, axial patterning, establishment and migration of primordial germ cells, early neurulation, blood island differentiation, yolk sac and ectoplacental cone development, among others. The combination of the ex utero culture platform with the amenability and scalability of SEMs that can bypass the use of mice, oocytes and zygotes represents a unique strategy to investigate how cells are assembled into embryos from bottom-up.
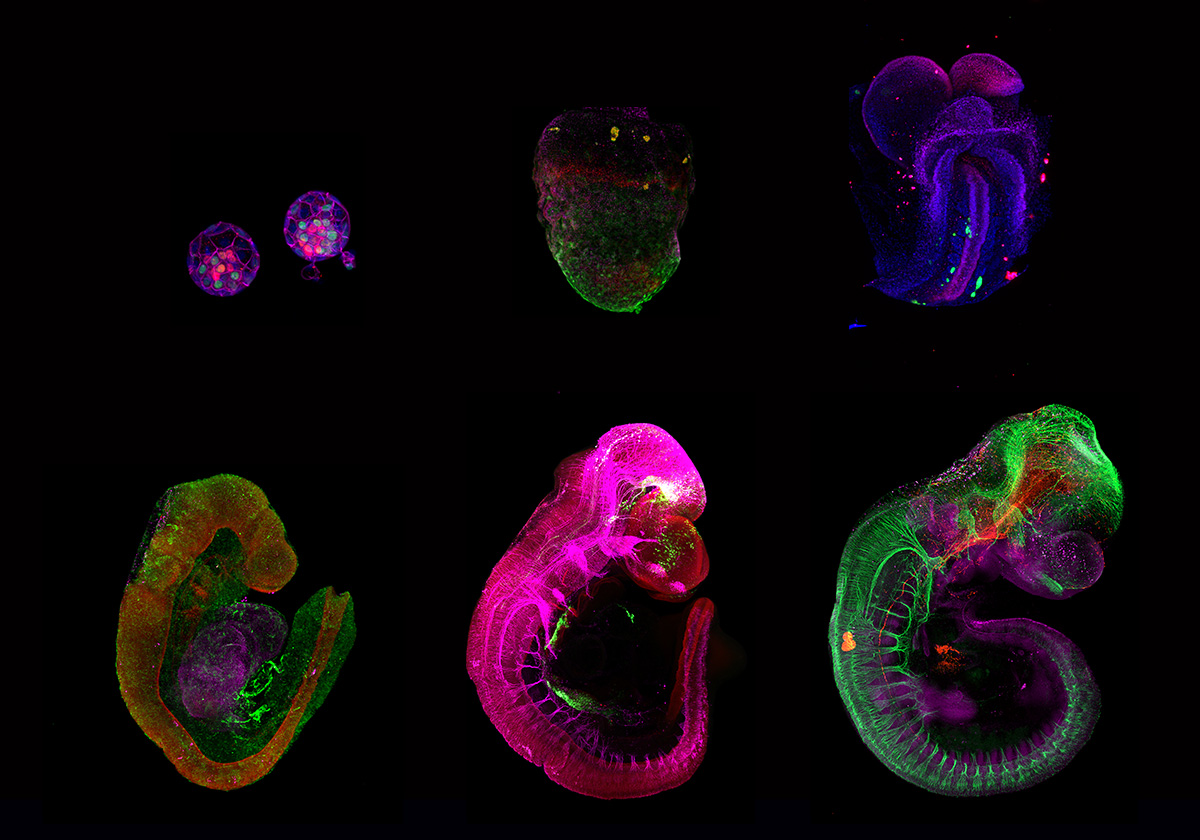
Experimental perturbations of developing embryos
Besides our interest on devising artificial conditions and platforms for reconstituting growth of mammalian embryos outside the uterine environment, we aim to take advantage of these ex utero systems to investigate fundamental questions during normal and perturbed embryogenesis. We employ embryos isolated from natural matings as well as embryo models assembled from pluripotent stem cells as two experimental systems to explore complementary questions in developmental and stem cell biology. We aim to combine the ex utero culture with cutting-edge technologies such as lineage tracing, optogenetics, CRISPR/Cas9, single cell genomics and proteomics, as well as the unique microscopy methods available at Janelia in order to trace and manipulate embryogenesis.
We expect our ex utero platforms for natural and stem cell-derived embryos to serve as a guide for engineering similar culture systems for other species, such as non-human primates and human embryos. Besides, we believe that only stringent comparison of embryo models to natural embryos will help elucidating to what extent embryo models resemble natural embryo development, as well as which processes might represent in vitro artifacts inherent to these models.
As part of the new 4D Cellular Physiology department at HHMI Janelia, we aim to work together with other Janelia labs to improve our understanding of how cells and tissues are built and maintained across organisms.
