Main Menu (Mobile)- Block
- Overview
-
Support Teams
- Overview
- Anatomy and Histology
- Cryo-Electron Microscopy
- Electron Microscopy
- Flow Cytometry
- Gene Targeting and Transgenics
- High Performance Computing
- Immortalized Cell Line Culture
- Integrative Imaging
- Invertebrate Shared Resource
- Janelia Experimental Technology
- Mass Spectrometry
- Media Prep
- Molecular Genomics
- Primary & iPS Cell Culture
- Project Pipeline Support
- Project Technical Resources
- Quantitative Genomics
- Scientific Computing
- Viral Tools
- Vivarium
- Open Science
- You + Janelia
- About Us
Main Menu - Block
- Overview
- Anatomy and Histology
- Cryo-Electron Microscopy
- Electron Microscopy
- Flow Cytometry
- Gene Targeting and Transgenics
- High Performance Computing
- Immortalized Cell Line Culture
- Integrative Imaging
- Invertebrate Shared Resource
- Janelia Experimental Technology
- Mass Spectrometry
- Media Prep
- Molecular Genomics
- Primary & iPS Cell Culture
- Project Pipeline Support
- Project Technical Resources
- Quantitative Genomics
- Scientific Computing
- Viral Tools
- Vivarium
Our research will focus on ion channels in the nervous system, especially in the control of cell signaling pathways, and the structures of the molecules in those pathways.
Lab Updates
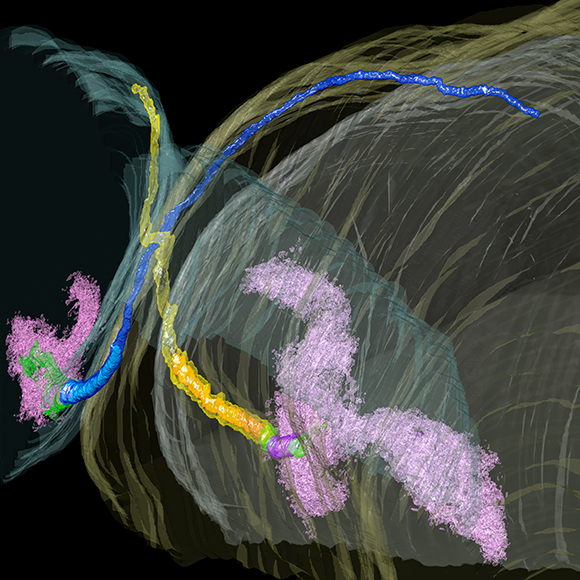
In July of 2017, the Clapham laboratory moved to the Janelia Research Campus. Here, we will continue work on the ion channels and calcium signals. We have two major interests at present. First, we are interested in the ion channels in primary cilia and how they affect signaling pathways, such as Hedgehog, in embryonic and mature neurons and glia of the hippocampus. We are also pursuing the cryoEM structures of the two main channels of primary cilia so that we can understand their gating.
Second, we are interested in understanding the two unusual ion channels, TRPM7 and TRPM6. These channels are in all cells, and when genetically deleted, are lethal. The channels are unique in that they possess active kinase domains that when cleaved, enter the nucleus and phosphorylate distinct residues on histones that control chromatin accessibility and thus transcription. Answering these questions will require electrophysiology, advanced light microscopy, mouse genetics, and structural approaches.