Filter
Associated Lab
- Ahrens Lab (1) Apply Ahrens Lab filter
- Aso Lab (40) Apply Aso Lab filter
- Betzig Lab (1) Apply Betzig Lab filter
- Bock Lab (2) Apply Bock Lab filter
- Branson Lab (4) Apply Branson Lab filter
- Cardona Lab (1) Apply Cardona Lab filter
- Clapham Lab (1) Apply Clapham Lab filter
- Funke Lab (2) Apply Funke Lab filter
- Harris Lab (1) Apply Harris Lab filter
- Heberlein Lab (1) Apply Heberlein Lab filter
- Hess Lab (2) Apply Hess Lab filter
- Jayaraman Lab (1) Apply Jayaraman Lab filter
- Lippincott-Schwartz Lab (1) Apply Lippincott-Schwartz Lab filter
- Rubin Lab (30) Apply Rubin Lab filter
- Saalfeld Lab (1) Apply Saalfeld Lab filter
- Scheffer Lab (2) Apply Scheffer Lab filter
- Simpson Lab (1) Apply Simpson Lab filter
- Truman Lab (1) Apply Truman Lab filter
- Turner Lab (7) Apply Turner Lab filter
- Zlatic Lab (1) Apply Zlatic Lab filter
Associated Project Team
Publication Date
- 2025 (1) Apply 2025 filter
- 2024 (3) Apply 2024 filter
- 2023 (6) Apply 2023 filter
- 2022 (1) Apply 2022 filter
- 2021 (1) Apply 2021 filter
- 2020 (3) Apply 2020 filter
- 2019 (3) Apply 2019 filter
- 2018 (4) Apply 2018 filter
- 2017 (3) Apply 2017 filter
- 2016 (2) Apply 2016 filter
- 2015 (6) Apply 2015 filter
- 2014 (3) Apply 2014 filter
- 2013 (1) Apply 2013 filter
- 2012 (2) Apply 2012 filter
- 2011 (1) Apply 2011 filter
Type of Publication
40 Publications
Showing 31-40 of 40 resultsMemory guides the choices an animal makes across widely varying conditions in dynamic environments. Consequently, the most adaptive choice depends on the options available. How can a single memory support optimal behavior across different sets of choice options? We address this using olfactory learning in Drosophila. Even when we restrict an odor-punishment association to a single set of synapses using optogenetics, we find that flies still show choice behavior that depends on the options it encounters. Here we show that how the odor choices are presented to the animal influences memory recall itself. Presenting two similar odors in sequence enabled flies to not only discriminate them behaviorally but also at the level of neural activity. However, when the same odors were encountered as solitary stimuli, no such differences were detectable. These results show that memory recall is not simply a comparison to a static learned template, but can be adaptively modulated by stimulus dynamics.
Although all sensory circuits ascend to higher brain areas where stimuli are represented in sparse, stimulus-specific activity patterns, relatively little is known about sensory coding on the descending side of neural circuits, as a network converges. In insects, mushroom bodies have been an important model system for studying sparse coding in the olfactory system, where this format is important for accurate memory formation. In Drosophila, it has recently been shown that the 2,000 Kenyon cells of the mushroom body converge onto a population of only 34 mushroom body output neurons (MBONs), which fall into 21 anatomically distinct cell types. Here we provide the first, to our knowledge, comprehensive view of olfactory representations at the fourth layer of the circuit, where we find a clear transition in the principles of sensory coding. We show that MBON tuning curves are highly correlated with one another. This is in sharp contrast to the process of progressive decorrelation of tuning in the earlier layers of the circuit. Instead, at the population level, odour representations are reformatted so that positive and negative correlations arise between representations of different odours. At the single-cell level, we show that uniquely identifiable MBONs display profoundly different tuning across different animals, but that tuning of the same neuron across the two hemispheres of an individual fly was nearly identical. Thus, individualized coordination of tuning arises at this level of the olfactory circuit. Furthermore, we find that this individualization is an active process that requires a learning-related gene, rutabaga. Ultimately, neural circuits have to flexibly map highly stimulus-specific information in sparse layers onto a limited number of different motor outputs. The reformatting of sensory representations we observe here may mark the beginning of this sensory-motor transition in the olfactory system.
The Drosophila mushroom body (MB) is a key associative memory center that has also been implicated in the control of sleep. However, the identity of MB neurons underlying homeostatic sleep regulation, as well as the types of sleep signals generated by specific classes of MB neurons, has remained poorly understood. We recently identified two MB output neuron (MBON) classes whose axons convey sleep control signals from the MB to converge in the same downstream target region: a cholinergic sleep-promoting MBON class and a glutamatergic wake-promoting MBON class. Here, we deploy a combination of neurogenetic, behavioral, and physiological approaches to identify and mechanistically dissect sleep-controlling circuits of the MB. Our studies reveal the existence of two segregated excitatory synaptic microcircuits that propagate homeostatic sleep information from different populations of intrinsic MB "Kenyon cells" (KCs) to specific sleep-regulating MBONs: sleep-promoting KCs increase sleep by preferentially activating the cholinergic MBONs, while wake-promoting KCs decrease sleep by preferentially activating the glutamatergic MBONs. Importantly, activity of the sleep-promoting MB microcircuit is increased by sleep deprivation and is necessary for homeostatic rebound sleep (i.e., the increased sleep that occurs after, and in compensation for, sleep lost during deprivation). These studies reveal for the first time specific functional connections between subsets of KCs and particular MBONs and establish the identity of synaptic microcircuits underlying transmission of homeostatic sleep signals in the MB.
Painful events establish opponent memories: cues that precede pain are remembered negatively, whereas cues that follow pain, thus coinciding with relief are recalled positively. How do individual reinforcement-signaling neurons contribute to this "timing-dependent valence-reversal?" We addressed this question using an optogenetic approach in the fruit fly. Two types of fly dopaminergic neuron, each comprising just one paired cell, indeed established learned avoidance of odors that preceded their photostimulation during training, and learned approach to odors that followed the photostimulation. This is in striking parallel to punishment versus relief memories reinforced by a real noxious event. For only one of these neuron types, both effects were strong enough for further analyses. Notably, interfering with dopamine biosynthesis in these neurons partially impaired the punishing effect, but not the relieving after-effect of their photostimulation. We discuss how this finding constraints existing computational models of punishment versus relief memories and introduce a new model, which also incorporates findings from mammals. Furthermore, whether using dopaminergic neuron photostimulation or a real noxious event, more prolonged punishment led to stronger relief. This parametric feature of relief may also apply to other animals and may explain particular aspects of related behavioral dysfunction in humans.
Animals exhibit a behavioral response to novel sensory stimuli about which they have no prior knowledge. We have examined the neural and behavioral correlates of novelty and familiarity in the olfactory system of Drosophila. Novel odors elicit strong activity in output neurons (MBONs) of the α'3 compartment of the mushroom body that is rapidly suppressed upon repeated exposure to the same odor. This transition in neural activity upon familiarization requires odor-evoked activity in the dopaminergic neuron innervating this compartment. Moreover, exposure of a fly to novel odors evokes an alerting response that can also be elicited by optogenetic activation of α'3 MBONs. Silencing these MBONs eliminates the alerting behavior. These data suggest that the α'3 compartment plays a causal role in the behavioral response to novel and familiar stimuli as a consequence of dopamine-mediated plasticity at the Kenyon cell-MBONα'3 synapse.
Dopamine signals reward in animal brains. A single presentation of a sugar reward to Drosophila activates distinct subsets of dopamine neurons that independently induce short- and long-term olfactory memories (STM and LTM, respectively). In this study, we show that a recurrent reward circuit underlies the formation and consolidation of LTM. This feedback circuit is composed of a single class of reward-signaling dopamine neurons (PAM-α1) projecting to a restricted region of the mushroom body (MB), and a specific MB output cell type, MBON-α1, whose dendrites arborize that same MB compartment. Both MBON-α1 and PAM-α1 neurons are required during the acquisition and consolidation of appetitive LTM. MBON-α1 additionally mediates the retrieval of LTM, which is dependent on the dopamine receptor signaling in the MB α/β neurons. Our results suggest that a reward signal transforms a nascent memory trace into a stable LTM using a feedback circuit at the cost of memory specificity.
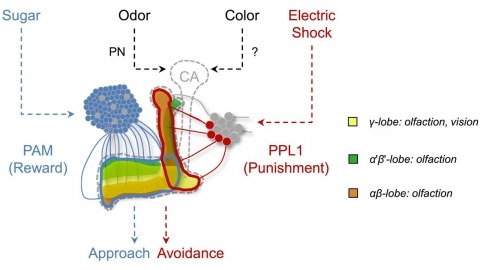
In nature, animals form memories associating reward or punishment with stimuli from different sensory modalities, such as smells and colors. It is unclear, however, how distinct sensory memories are processed in the brain. We established appetitive and aversive visual learning assays for Drosophila that are comparable to the widely used olfactory learning assays. These assays share critical features, such as reinforcing stimuli (sugar reward and electric shock punishment), and allow direct comparison of the cellular requirements for visual and olfactory memories. We found that the same subsets of dopamine neurons drive formation of both sensory memories. Furthermore, distinct yet partially overlapping subsets of mushroom body intrinsic neurons are required for visual and olfactory memories. Thus, our results suggest that distinct sensory memories are processed in a common brain center. Such centralization of related brain functions is an economical design that avoids the repetition of similar circuit motifs.
Making inferences about the computations performed by neuronal circuits from synapse-level connectivity maps is an emerging opportunity in neuroscience. The mushroom body (MB) is well positioned for developing and testing such an approach due to its conserved neuronal architecture, recently completed dense connectome, and extensive prior experimental studies of its roles in learning, memory and activity regulation. Here we identify new components of the MB circuit in , including extensive visual input and MB output neurons (MBONs) with direct connections to descending neurons. We find unexpected structure in sensory inputs, in the transfer of information about different sensory modalities to MBONs, and in the modulation of that transfer by dopaminergic neurons (DANs). We provide insights into the circuitry used to integrate MB outputs, connectivity between the MB and the central complex and inputs to DANs, including feedback from MBONs. Our results provide a foundation for further theoretical and experimental work.
We identified the neurons comprising the Drosophila mushroom body (MB), an associative center in invertebrate brains, and provide a comprehensive map describing their potential connections. Each of the 21 MB output neuron (MBON) types elaborates segregated dendritic arbors along the parallel axons of ∼2000 Kenyon cells, forming 15 compartments that collectively tile the MB lobes. MBON axons project to five discrete neuropils outside of the MB and three MBON types form a feedforward network in the lobes. Each of the 20 dopaminergic neuron (DAN) types projects axons to one, or at most two, of the MBON compartments. Convergence of DAN axons on compartmentalized Kenyon cell-MBON synapses creates a highly ordered unit that can support learning to impose valence on sensory representations. The elucidation of the complement of neurons of the MB provides a comprehensive anatomical substrate from which one can infer a functional logic of associative olfactory learning and memory.
The Mushroom Body (MB) is the primary location of stored associative memories in the Drosophila brain. We discuss recent advances in understanding the MB's neuronal circuits made using advanced light microscopic methods and cell-type-specific genetic tools. We also review how the compartmentalized nature of the MB's organization allows this brain area to form and store memories with widely different dynamics.