Filter
Associated Lab
- Aguilera Castrejon Lab (17) Apply Aguilera Castrejon Lab filter
- Ahrens Lab (69) Apply Ahrens Lab filter
- Aso Lab (42) Apply Aso Lab filter
- Baker Lab (38) Apply Baker Lab filter
- Betzig Lab (115) Apply Betzig Lab filter
- Beyene Lab (14) Apply Beyene Lab filter
- Bock Lab (17) Apply Bock Lab filter
- Branson Lab (54) Apply Branson Lab filter
- Card Lab (43) Apply Card Lab filter
- Cardona Lab (64) Apply Cardona Lab filter
- Chklovskii Lab (13) Apply Chklovskii Lab filter
- Clapham Lab (15) Apply Clapham Lab filter
- Cui Lab (19) Apply Cui Lab filter
- Darshan Lab (12) Apply Darshan Lab filter
- Dennis Lab (2) Apply Dennis Lab filter
- Dickson Lab (46) Apply Dickson Lab filter
- Druckmann Lab (25) Apply Druckmann Lab filter
- Dudman Lab (53) Apply Dudman Lab filter
- Eddy/Rivas Lab (30) Apply Eddy/Rivas Lab filter
- Egnor Lab (11) Apply Egnor Lab filter
- Espinosa Medina Lab (21) Apply Espinosa Medina Lab filter
- Feliciano Lab (10) Apply Feliciano Lab filter
- Fetter Lab (41) Apply Fetter Lab filter
- FIB-SEM Technology (1) Apply FIB-SEM Technology filter
- Fitzgerald Lab (29) Apply Fitzgerald Lab filter
- Freeman Lab (15) Apply Freeman Lab filter
- Funke Lab (42) Apply Funke Lab filter
- Gonen Lab (91) Apply Gonen Lab filter
- Grigorieff Lab (62) Apply Grigorieff Lab filter
- Harris Lab (64) Apply Harris Lab filter
- Heberlein Lab (94) Apply Heberlein Lab filter
- Hermundstad Lab (30) Apply Hermundstad Lab filter
- Hess Lab (79) Apply Hess Lab filter
- Ilanges Lab (3) Apply Ilanges Lab filter
- Jayaraman Lab (48) Apply Jayaraman Lab filter
- Ji Lab (33) Apply Ji Lab filter
- Johnson Lab (6) Apply Johnson Lab filter
- Kainmueller Lab (19) Apply Kainmueller Lab filter
- Karpova Lab (14) Apply Karpova Lab filter
- Keleman Lab (13) Apply Keleman Lab filter
- Keller Lab (76) Apply Keller Lab filter
- Koay Lab (18) Apply Koay Lab filter
- Lavis Lab (154) Apply Lavis Lab filter
- Lee (Albert) Lab (34) Apply Lee (Albert) Lab filter
- Leonardo Lab (23) Apply Leonardo Lab filter
- Li Lab (30) Apply Li Lab filter
- Lippincott-Schwartz Lab (178) Apply Lippincott-Schwartz Lab filter
- Liu (Yin) Lab (7) Apply Liu (Yin) Lab filter
- Liu (Zhe) Lab (64) Apply Liu (Zhe) Lab filter
- Looger Lab (138) Apply Looger Lab filter
- Magee Lab (49) Apply Magee Lab filter
- Menon Lab (18) Apply Menon Lab filter
- Murphy Lab (13) Apply Murphy Lab filter
- O'Shea Lab (7) Apply O'Shea Lab filter
- Otopalik Lab (13) Apply Otopalik Lab filter
- Pachitariu Lab (49) Apply Pachitariu Lab filter
- Pastalkova Lab (18) Apply Pastalkova Lab filter
- Pavlopoulos Lab (19) Apply Pavlopoulos Lab filter
- Pedram Lab (15) Apply Pedram Lab filter
- Podgorski Lab (16) Apply Podgorski Lab filter
- Reiser Lab (54) Apply Reiser Lab filter
- Riddiford Lab (44) Apply Riddiford Lab filter
- Romani Lab (49) Apply Romani Lab filter
- Rubin Lab (148) Apply Rubin Lab filter
- Saalfeld Lab (64) Apply Saalfeld Lab filter
- Satou Lab (16) Apply Satou Lab filter
- Scheffer Lab (38) Apply Scheffer Lab filter
- Schreiter Lab (69) Apply Schreiter Lab filter
- Sgro Lab (21) Apply Sgro Lab filter
- Shroff Lab (31) Apply Shroff Lab filter
- Simpson Lab (23) Apply Simpson Lab filter
- Singer Lab (80) Apply Singer Lab filter
- Spruston Lab (97) Apply Spruston Lab filter
- Stern Lab (158) Apply Stern Lab filter
- Sternson Lab (54) Apply Sternson Lab filter
- Stringer Lab (39) Apply Stringer Lab filter
- Svoboda Lab (135) Apply Svoboda Lab filter
- Tebo Lab (35) Apply Tebo Lab filter
- Tervo Lab (9) Apply Tervo Lab filter
- Tillberg Lab (21) Apply Tillberg Lab filter
- Tjian Lab (64) Apply Tjian Lab filter
- Truman Lab (88) Apply Truman Lab filter
- Turaga Lab (53) Apply Turaga Lab filter
- Turner Lab (39) Apply Turner Lab filter
- Vale Lab (8) Apply Vale Lab filter
- Voigts Lab (4) Apply Voigts Lab filter
- Wang (Meng) Lab (27) Apply Wang (Meng) Lab filter
- Wang (Shaohe) Lab (25) Apply Wang (Shaohe) Lab filter
- Wu Lab (9) Apply Wu Lab filter
- Zlatic Lab (28) Apply Zlatic Lab filter
- Zuker Lab (25) Apply Zuker Lab filter
Associated Project Team
- CellMap (12) Apply CellMap filter
- COSEM (3) Apply COSEM filter
- FIB-SEM Technology (5) Apply FIB-SEM Technology filter
- Fly Descending Interneuron (12) Apply Fly Descending Interneuron filter
- Fly Functional Connectome (14) Apply Fly Functional Connectome filter
- Fly Olympiad (5) Apply Fly Olympiad filter
- FlyEM (56) Apply FlyEM filter
- FlyLight (50) Apply FlyLight filter
- GENIE (47) Apply GENIE filter
- Integrative Imaging (7) Apply Integrative Imaging filter
- Larval Olympiad (2) Apply Larval Olympiad filter
- MouseLight (18) Apply MouseLight filter
- NeuroSeq (1) Apply NeuroSeq filter
- ThalamoSeq (1) Apply ThalamoSeq filter
- Tool Translation Team (T3) (28) Apply Tool Translation Team (T3) filter
- Transcription Imaging (49) Apply Transcription Imaging filter
Publication Date
- 2025 (227) Apply 2025 filter
- 2024 (212) Apply 2024 filter
- 2023 (158) Apply 2023 filter
- 2022 (192) Apply 2022 filter
- 2021 (194) Apply 2021 filter
- 2020 (196) Apply 2020 filter
- 2019 (202) Apply 2019 filter
- 2018 (232) Apply 2018 filter
- 2017 (217) Apply 2017 filter
- 2016 (209) Apply 2016 filter
- 2015 (252) Apply 2015 filter
- 2014 (236) Apply 2014 filter
- 2013 (194) Apply 2013 filter
- 2012 (190) Apply 2012 filter
- 2011 (190) Apply 2011 filter
- 2010 (161) Apply 2010 filter
- 2009 (158) Apply 2009 filter
- 2008 (140) Apply 2008 filter
- 2007 (106) Apply 2007 filter
- 2006 (92) Apply 2006 filter
- 2005 (67) Apply 2005 filter
- 2004 (57) Apply 2004 filter
- 2003 (58) Apply 2003 filter
- 2002 (39) Apply 2002 filter
- 2001 (28) Apply 2001 filter
- 2000 (29) Apply 2000 filter
- 1999 (14) Apply 1999 filter
- 1998 (18) Apply 1998 filter
- 1997 (16) Apply 1997 filter
- 1996 (10) Apply 1996 filter
- 1995 (18) Apply 1995 filter
- 1994 (12) Apply 1994 filter
- 1993 (10) Apply 1993 filter
- 1992 (6) Apply 1992 filter
- 1991 (11) Apply 1991 filter
- 1990 (11) Apply 1990 filter
- 1989 (6) Apply 1989 filter
- 1988 (1) Apply 1988 filter
- 1987 (7) Apply 1987 filter
- 1986 (4) Apply 1986 filter
- 1985 (5) Apply 1985 filter
- 1984 (2) Apply 1984 filter
- 1983 (2) Apply 1983 filter
- 1982 (3) Apply 1982 filter
- 1981 (3) Apply 1981 filter
- 1980 (1) Apply 1980 filter
- 1979 (1) Apply 1979 filter
- 1976 (2) Apply 1976 filter
- 1973 (1) Apply 1973 filter
- 1970 (1) Apply 1970 filter
- 1967 (1) Apply 1967 filter
Type of Publication
4202 Publications
Showing 3931-3940 of 4202 resultsStarvation induces a protective process of self-cannibalization called autophagy that is thought to mediate nonselective degradation of cytoplasmic material. We recently reported that mitochondria escape autophagosomal degradation through extensive fusion into mitochondrial networks upon certain starvation conditions. The extent of mitochondrial elongation is dependent on the type of nutrient deprivation, with amino acid depletion having a particularly strong effect. Downregulation of the mitochondrial fission protein Drp1 was determined to be important in bringing about starvation-induced mitochondrial fusion. The formation of mitochondrial networks during nutrient depletion selectively blocked their autophagic degradation, presumably allowing cells to sustain efficient ATP production and thereby survive starvation.
One of the key morphogenetic processes used during development is the controlled intercalation of cells between their neighbors. This process has been co-opted into a range of developmental events, and it also underlies an event that occurs in each major group of bilaterians: elongation of the embryo along the anterior-posterior axis [1]. In Drosophila, a novel component of this process was recently discovered by Paré et al., who showed that three Toll genes function together to drive cell intercalation during germband extension [2]. This finding raises the question of whether this role of Toll genes is an evolutionary novelty of flies or a general mechanism of embryonic morphogenesis. Here we show that the Toll gene function in axis elongation is, in fact, widely conserved among arthropods. First, we functionally demonstrate that two Toll genes are required for cell intercalation in the beetle Tribolium castaneum. We then show that these genes belong to a previously undescribed Toll subfamily and that members of this subfamily exhibit striped expression (as seen in Tribolium and previously reported in Drosophila [3-5]) in embryos of six other arthropod species spanning the entire phylum. Last, we show that two of these Toll genes are required for normal morphogenesis during anterior-posterior embryo elongation in the spider Parasteatoda tepidariorum, a member of the most basally branching arthropod lineage. From our findings, we hypothesize that Toll genes had a morphogenetic function in embryo elongation in the last common ancestor of all arthropods, which existed over 550 million years ago.
In mammals, fat store levels are regulated by brain centers that control food intake and metabolism. A new study by Al-Anzi and colleagues in this issue of Neuron identifies neurons with similar functions in Drosophila, further establishing the fly as a legitimate model to study obesity.
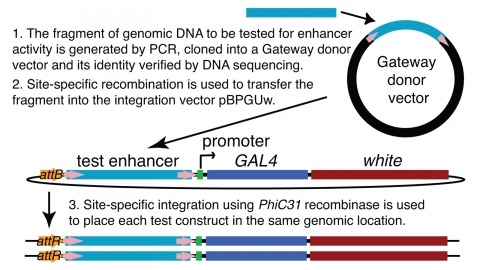
We demonstrate the feasibility of generating thousands of transgenic Drosophila melanogaster lines in which the expression of an exogenous gene is reproducibly directed to distinct small subsets of cells in the adult brain. We expect the expression patterns produced by the collection of 5,000 lines that we are currently generating to encompass all neurons in the brain in a variety of intersecting patterns. Overlapping 3-kb DNA fragments from the flanking noncoding and intronic regions of genes thought to have patterned expression in the adult brain were inserted into a defined genomic location by site-specific recombination. These fragments were then assayed for their ability to function as transcriptional enhancers in conjunction with a synthetic core promoter designed to work with a wide variety of enhancer types. An analysis of 44 fragments from four genes found that >80% drive expression patterns in the brain; the observed patterns were, on average, comprised of <100 cells. Our results suggest that the D. melanogaster genome contains >50,000 enhancers and that multiple enhancers drive distinct subsets of expression of a gene in each tissue and developmental stage. We expect that these lines will be valuable tools for neuroanatomy as well as for the elucidation of neuronal circuits and information flow in the fly brain.
Sparse manipulation of neuron excitability during free behavior is critical for identifying neural substrates of behavior. Genetic tools for precise neuronal manipulation exist in the fruit fly, Drosophila melanogaster, but behavioral tools are still lacking to identify potentially subtle phenotypes only detectible using high-throughput and high spatiotemporal resolution. We developed three assay components that can be used modularly to study natural and optogenetically induced behaviors. FlyGate automatically releases flies one at a time into an assay. FlyDetect tracks flies in real time, is robust to severe occlusions, and can be used to track appendages, such as the head. GlobeDisplay is a spherical projection system covering the fly's visual receptive field with a single projector. We demonstrate the utility of these components in an integrated system, FlyPEZ, by comprehensively modeling the input-output function for directional looming-evoked escape takeoffs and describing a millisecond-timescale phenotype from genetic silencing of a single visual projection neuron type.
In the hypothalamic arcuate nucleus (ARC), pro-opiomelanocortin (POMC) neurons inhibit feeding and neuropeptide-Y (NPY) neurons stimulate feeding. We tested whether neurons in the ventromedial hypothalamic nucleus (VMH), a known satiety center, activate anorexigenic neuronal pathways in the ARC by projecting either excitatory synaptic inputs to POMC neurons and/or inhibitory inputs to NPY neurons. Using laser scanning photostimulation in brain slices from transgenic mice, we found that POMC and NPY neurons, which are interspersed in the ARC, are nevertheless regulated by anatomically distinct synaptic inputs. POMC neurons received strong excitatory input from the medial VMH (mVMH), whereas NPY neurons did not and, instead, received weak inhibitory input only from within the ARC. The strength of the excitatory input from the mVMH to POMC neurons was diminished by fasting. These data identify a new molecularly defined circuit that is dynamically regulated by nutritional state in a manner consistent with the known role of the VMH as a satiety center.
The striatum shows general topographic organization and regional differences in behavioral functions. How corticostriatal topography differs across cortical areas and cell types to support these distinct functions is unclear. This study contrasted corticostriatal projections from two layer 5 cell types, intratelencephalic (IT-type) and pyramidal tract (PT-type) neurons, using viral vectors expressing fluorescent reporters in Cre-driver mice. Corticostriatal projections from sensory and motor cortex are somatotopic, with a decreasing topographic specificity as injection sites move from sensory to motor and frontal areas. Topographic organization differs between IT-type and PT-type neurons, including injections in the same site, with IT-type neurons having higher topographic stereotypy than PT-type neurons. Furthermore, IT-type projections from interconnected cortical areas have stronger correlations in corticostriatal targeting than PT-type projections do. As predicted by a longstanding model, corticostriatal projections of interconnected cortical areas form parallel circuits in the basal ganglia.
Insects and mammals share similarities of neural organization underlying the perception of odors, taste, vision, sound, and gravity. We observed that insect somatosensation also corresponds to that of mammals. In Drosophila, the projections of all the somatosensory neuron types to the insect's equivalent of the spinal cord segregated into modality-specific layers comparable to those in mammals. Some sensory neurons innervate the ventral brain directly to form modality-specific and topological somatosensory maps. Ascending interneurons with dendrites in matching layers of the nerve cord send axons that converge to respective brain regions. Pathways arising from leg somatosensory neurons encode distinct qualities of leg movement information and play different roles in ground detection. Establishment of the ground pattern and genetic tools for neuronal manipulation should provide the basis for elucidating the mechanisms underlying somatosensation.
The medial entorhinal cortex is part of a neural system for mapping the position of an individual within a physical environment. Grid cells, a key component of this system, fire in a characteristic hexagonal pattern of locations, and are organized in modules that collectively form a population code for the animal's allocentric position. The invariance of the correlation structure of this population code across environments and behavioural states, independent of specific sensory inputs, has pointed to intrinsic, recurrently connected continuous attractor networks (CANs) as a possible substrate of the grid pattern. However, whether grid cell networks show continuous attractor dynamics, and how they interface with inputs from the environment, has remained unclear owing to the small samples of cells obtained so far. Here, using simultaneous recordings from many hundreds of grid cells and subsequent topological data analysis, we show that the joint activity of grid cells from an individual module resides on a toroidal manifold, as expected in a two-dimensional CAN. Positions on the torus correspond to positions of the moving animal in the environment. Individual cells are preferentially active at singular positions on the torus. Their positions are maintained between environments and from wakefulness to sleep, as predicted by CAN models for grid cells but not by alternative feedforward models. This demonstration of network dynamics on a toroidal manifold provides a population-level visualization of CAN dynamics in grid cells.