Filter
Associated Lab
- Aso Lab (1) Apply Aso Lab filter
- Branson Lab (1) Apply Branson Lab filter
- Card Lab (4) Apply Card Lab filter
- Cardona Lab (17) Apply Cardona Lab filter
- Dickson Lab (1) Apply Dickson Lab filter
- Fetter Lab (9) Apply Fetter Lab filter
- Heberlein Lab (1) Apply Heberlein Lab filter
- Riddiford Lab (17) Apply Riddiford Lab filter
- Rubin Lab (4) Apply Rubin Lab filter
- Simpson Lab (2) Apply Simpson Lab filter
- Singer Lab (1) Apply Singer Lab filter
- Stern Lab (4) Apply Stern Lab filter
- Truman Lab (88) Apply Truman Lab filter
- Zlatic Lab (13) Apply Zlatic Lab filter
Associated Project Team
Publication Date
- 2023 (2) Apply 2023 filter
- 2021 (3) Apply 2021 filter
- 2020 (3) Apply 2020 filter
- 2019 (4) Apply 2019 filter
- 2018 (8) Apply 2018 filter
- 2017 (6) Apply 2017 filter
- 2016 (11) Apply 2016 filter
- 2015 (6) Apply 2015 filter
- 2014 (1) Apply 2014 filter
- 2013 (3) Apply 2013 filter
- 2012 (3) Apply 2012 filter
- 2011 (1) Apply 2011 filter
- 2010 (4) Apply 2010 filter
- 2009 (4) Apply 2009 filter
- 2008 (2) Apply 2008 filter
- 2007 (1) Apply 2007 filter
- 2006 (3) Apply 2006 filter
- 2005 (2) Apply 2005 filter
- 2004 (2) Apply 2004 filter
- 2003 (1) Apply 2003 filter
- 2002 (1) Apply 2002 filter
- 2001 (2) Apply 2001 filter
- 2000 (2) Apply 2000 filter
- 1999 (1) Apply 1999 filter
- 1998 (2) Apply 1998 filter
- 1997 (2) Apply 1997 filter
- 1996 (1) Apply 1996 filter
- 1994 (1) Apply 1994 filter
- 1991 (1) Apply 1991 filter
- 1990 (1) Apply 1990 filter
- 1987 (1) Apply 1987 filter
- 1986 (1) Apply 1986 filter
- 1976 (1) Apply 1976 filter
- 1970 (1) Apply 1970 filter
Type of Publication
88 Publications
Showing 51-60 of 88 resultsIn insects, the neuropeptide eclosion hormone (EH) acts on the CNS to evoke the stereotyped behaviors that cause ecdysis, the shedding of the cuticle at the end of each molt. Concomitantly, EH induces an increase in cyclic GMP (cGMP). Using antibodies against this second messenger, we show that this increase is confined to a network of 50 peptidergic neurons distributed throughout the CNS. Increases appeared 30 min after EH treatment, spread rapidly throughout these neurons, and were extremely long lived. We show that this response is synaptically driven, and does not involve the soluble, nitric oxide (NO)-activated, guanylate cyclase. Stereotyped variations in the duration of the cGMP response among neurons suggest a role in coordinating responses having different latencies and durations.
The vast majority of the adult fly ventral nerve cord is composed of 34 hemilineages, which are clusters of lineally related neurons. Neurons in these hemilineages use one of the three fast-acting neurotransmitters (acetylcholine, GABA, or glutamate) for communication. We generated a comprehensive neurotransmitter usage map for the entire ventral nerve cord. We did not find any cases of neurons using more than one neurotransmitter, but found that the acetylcholine specific gene ChAT is transcribed in many glutamatergic and GABAergic neurons, but these transcripts typically do not leave the nucleus and are not translated. Importantly, our work uncovered a simple rule: All neurons within a hemilineage use the same neurotransmitter. Thus, neurotransmitter identity is acquired at the stem cell level. Our detailed transmitter- usage/lineage identity map will be a great resource for studying the developmental basis of behavior and deciphering how neuronal circuits function to regulate behavior.
Many developing insect neurones pass through a phase when they respond to nitric oxide (NO) by producing cyclic GMP. Studies on identified grasshopper motoneurones show that this NO sensitivity appears after the growth cone has arrived at its target but before it has started to send out branches. NO sensitivity typically ends as synaptogenesis is nearing completion. Data from interneurones and sensory neurones are also consistent with the hypothesis that NO sensitivity appears as a developing neurone changes from axonal outgrowth to maturation and synaptogenesis. Cyclic GMP likely constitutes part of a retrograde signalling pathway between a neurone and its synaptic partner. NO sensitivity also appears in some mature neurones at times when they may be undergoing synaptic rearrangement. Comparative studies on other insects indicate that the association between an NO-sensitive guanylate cyclase and synaptogenesis is an ancient one, as evidenced by its presence in both ancient and more recently evolved insect groups.
Visual systems transduce, process and transmit light-dependent environmental cues. Computation of visual features depends on photoreceptor neuron types (PR) present, organization of the eye and wiring of the underlying neural circuit. Here, we describe the circuit architecture of the visual system of Drosophila larvae by mapping the synaptic wiring diagram and neurotransmitters. By contacting different targets, the two larval PR-subtypes create two converging pathways potentially underlying the computation of ambient light intensity and temporal light changes already within this first visual processing center. Locally processed visual information then signals via dedicated projection interneurons to higher brain areas including the lateral horn and mushroom body. The stratified structure of the larval optic neuropil (LON) suggests common organizational principles with the adult fly and vertebrate visual systems. The complete synaptic wiring diagram of the LON paves the way to understanding how circuits with reduced numerical complexity control wide ranges of behaviors.
Insect molting is triggered by ecdysteroids, which are produced in the prothoracic glands (PG). The broad (br) gene is one of the ’early genes’ directly regulated by ecdysteroids. Ectopic expression of the BR-Z3 isoform in early second instar Drosophila larvae (L2) before the rise of the ecdysteroid titer prevented molting to the third instar, but the larvae subsequently formed L2 prepupae after prolonged feeding. When these larvae were fed on diet containing 20-hydroxyecdysone (20E), they formed pharate third instar larvae. The critical weight for normal L3 pupariation of w(1118) larvae was found to be 0.8 mg and that for L2 pupariation was 0.45 mg. We also defined a threshold weight for metamorphosis of 0.3 mg, above which L2 larvae will metamorphose when provided with 20E. BR-Z3 apparently works through the PG cells of the ring gland but not the putative neurosecretory cells that drive ecdysone secretion, because ectopic expression of BR-Z3 specifically in the ring gland caused 53% of the larvae to become permanent first instar larvae. Driving other BR isoforms in the ring gland prevented larval molting or pupariation to varying degrees. These molting defects were rescued by feeding 20E. Overexpression of each of the BR isoforms caused degeneration of the PG cells but on different time courses, indicating that BR is a signal for the degeneration of the PG cells that normally occurs during the pupal-adult transition.
During larval life most of the thoracic neuroblasts (NBs) in Drosophila undergo a second phase of neurogenesis to generate adult-specific neurons that remain in an immature, developmentally stalled state until pupation. Using a combination of MARCM and immunostaining with a neurotactin antibody Truman et al. (2004) identified 24 adult specific NB lineages within each thoracic hemineuromere of the larval ventral nervous system (VNS) but because the neurotactin labeling of lineage tracts disappearing early in metamorphosis they were unable extend the identification of the these lineages into the adult. Here we show that immunostaining with an antibody against the cell adhesion molecule Neuroglian reveals the same larval secondary lineage projections through metamorphosis and by identifying each neuroglian positive tract at selected stages we have traced the larval hemilineage tracts for all three thoracic neuromeres through metamorphosis into the adult. To validate tract identifications we used the genetic toolkit developed by Harris et al. (2015) to preserve hemilineage specific GAL4 expression patterns from larval into the adult stage. The immortalized expression proved a powerful confirmation of the analysis of the neuroglian scaffold. This work has enabled us to directly link the secondary, larval NB lineages to their adult counterparts. The data provide an anatomical framework that 1) makes it possible to assign most neurons to their parent lineage and 2) allows more precise definitions of the neuronal organization of the adult VNS based in developmental units/rules. This article is protected by copyright. All rights reserved.
The tobacco hornworm Manduca sexta exhibits dramatic changes in its body morphology and behavior as it is transformed from a larva into an adult during metamorphosis. Accompanying these changes is an extensive reorganization of this moth’s central nervous system (CNS), which involves both the death and remodeling of subsets of larval neurons. We report here that the segmental ganglia of the larvae also contain a stereotyped array of identifiable neuronal stem cells (neuroblasts) that contribute over 2,000 cells to each thoracic ganglion and about 40-80 cells to each abdominal ganglion. The distribution of these neuroblasts varies in a segment specific manner. Dormant neuroblasts are found adjacent to the neuropil in late embryos and early first instar larvae. After the molt to the second instar, these cells enlarge and begin to divide. Through a series of asymmetrical divisions, each neuroblast generates a discrete nest of 10-90 progeny by the end of larval life. These progeny (the imaginal nest cells) are developmentally arrested at an early stage of differentiation and remain so until metamorphosis. At the onset of metamorphosis, a wave of cell death sweeps through the nests, the extent of the death being much greater within the abdominal nests than in the thoracic nests. The surviving imaginal nest cells then differentiate to become functional neurons that are incorporated into the adult CNS.
Programmed cell death occurs in the nervous and muscular system of newly emerged adult Drosophila melanogaster. Many of the abdominal muscles that were used for eclosion and wing-spreading behavior degenerate by 12 hr after eclosion. Related neurons in the ventral ganglion also die within the first 24 hr. Ligation experiments showed that the muscle breakdown is triggered by a signal from the anterior region, presumably the head, that occurs about 1 hr before adult emergence. The timing of this signal suggests that eclosion hormone may be involved. Although muscle death is triggered prior to ecdysis, it can be delayed, at least temporarily, by forcing the emerging flies to show a prolonged ecdysis behavior. In contrast to the muscles, the death of the neurons is triggered after emergence. The signal for neuronal degeneration is closely correlated with the initiation of wing inflation behavior. Ligation and digging experiments and behavioral manipulations that either blocked or delayed wing expansion behavior had a parallel effect in suppressing or delaying neuronal death.
Dopaminergic neurons (DANs) drive learning across the animal kingdom, but the upstream circuits that regulate their activity and thereby learning remain poorly understood. We provide a synaptic-resolution connectome of the circuitry upstream of all DANs in a learning center, the mushroom body of Drosophila larva. We discover afferent sensory pathways and a large population of neurons that provide feedback from mushroom body output neurons and link distinct memory systems (aversive and appetitive). We combine this with functional studies of DANs and their presynaptic partners and with comprehensive circuit modeling. We find that DANs compare convergent feedback from aversive and appetitive systems, which enables the computation of integrated predictions that may improve future learning. Computational modeling reveals that the discovered feedback motifs increase model flexibility and performance on learning tasks. Our study provides the most detailed view to date of biological circuit motifs that support associative learning.
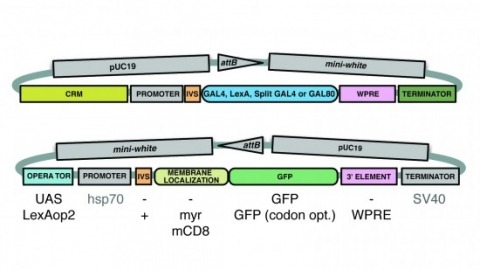
A wide variety of biological experiments rely on the ability to express an exogenous gene in a transgenic animal at a defined level and in a spatially and temporally controlled pattern. We describe major improvements of the methods available for achieving this objective in Drosophila melanogaster. We have systematically varied core promoters, UTRs, operator sequences, and transcriptional activating domains used to direct gene expression with the GAL4, LexA, and Split GAL4 transcription factors and the GAL80 transcriptional repressor. The use of site-specific integration allowed us to make quantitative comparisons between different constructs inserted at the same genomic location. We also characterized a set of PhiC31 integration sites for their ability to support transgene expression of both drivers and responders in the nervous system. The increased strength and reliability of these optimized reagents overcome many of the previous limitations of these methods and will facilitate genetic manipulations of greater complexity and sophistication.