Volumetric imaging in the near-infrared of neuronal activity of zebrafish larvae with WHaloCaMP. Credit: Farrants et al.
Eric Schreiter and Luke Lavis thought they had figured it out.
In 2021, the Janelia group leaders reported that they had developed a way to combine Schreiter’s engineered protein biosensors and Lavis’s bright, fluorescent Janelia Fluor dyes.
These sensors, which could track different physiological signals and brightly illuminate them in far-red light, would in theory enable scientists to perform imaging in live animals and track multiple physiological signals at the same time – two aspects of biological imaging that were difficult to do with existing sensors. Far-red light can penetrate deeper into tissues than other wavelengths, and it gives scientists an additional color to use outside the typical hues, like green and red, used in biological imaging.
“Everything was great, and it was fantastic, and we were happy – until we tried to use the sensors in animals, and it pretty much totally failed,” Schreiter recalls. “It was a bit of a bummer.”
Luckily, Helen Farrants had just arrived at Janelia for her postdoc in Schreiter’s lab, and she accepted the challenge of re-developing the protein biosensors to carry out their original intention.
Starting from scratch, Farrants created a new way for the engineered protein biosensors and the JF dyes to work together, enabling the team to accomplish their goal of measuring physiological signals in live animals. Their first proof-of-principle sensor, dubbed WHaloCaMP, can detect calcium signals – a key part of cellular communication – in live fruit flies, zebrafish, and mice.
The new technique can also be used to create a whole host of sensors for tracking other signals of interest. Being able to see these physiological signals in live animals could give biologists insight into how cells, tissues, and organs work together to carry out important functions.
“Helen started from scratch, from the ground up, and rebuilt this whole strategy for combining dyes and protein biosensors,” Schreiter says. “WHaloCaMP is the first demonstration, but it won’t be the last. It really is going to be a new general strategy in the field for making fluorescent biosensors to image physiology, especially in the far-red.”
Forging a new path
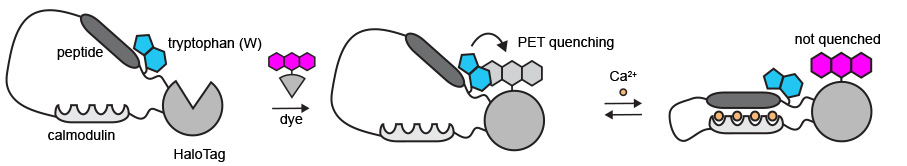
The main hurdle Farrants and the team had to overcome was figuring out a different way to make the protein biosensor and the JF dye work together.
The first sensors that the team built relied on dyes that change their form to become fluorescent. However, those dyes could not enter animal tissue – a problem that became apparent when the team tried to use the sensor in live animals and was unable to detect any signals.
After more than a year of trying different strategies, Farrants had the idea to use specific parts of the sensor protein to turn the fluorescence on and off, rather than using a change in the dye’s form. The team added an amino acid called tryptophan to the bioengineered protein sensor close to the attached dye. When the dye is in close contact with the tryptophan, the dye is turned off. In the presence of calcium, the protein changes shape – the tryptophan moves away from the dye, and the dye turns on.
“For a year and a half, nothing worked, but I remember the day that I made this tryptophan change and I saw just the tiniest change in fluorescence when I added calcium. I knew that we at least had a starting point – we had a hook,” Farrants recalls.
Three color functional multiplexed imaging of WHaloCaMP, jRGECO and iGlucoSnFr in zebrafish larva. Three colors are displayed in raw fluorescence together with an overly of fluorescence of all three channels. Credit: Farrants et al.
Seeing signals
Using tryptophan to modulate the dye’s fluorescence allowed the use of dyes that are easily taken up by tissues and used in live animals.
The researchers showed that WHaloCaMP could be used to detect calcium signals in live fruit flies, zebrafish, and mice. They also showed that it could be used alongside other sensors to detect up to three signals at the same time using distinct colors. In zebrafish, they showed that they could simultaneously detect glucose changes in cells, calcium signals in muscles, and calcium signals in neurons.
The team is now working with Janelia’s GENIE Project Team to develop an improved version of WHaloCaMP. They are also working with biologists at Janelia to use the new strategy to develop sensors for detecting other physiological signals and create sensors with additional JF dyes. The team has made their strategy for building the biosensors available to the broader research community as well, and other groups have started to develop additional versions of the sensors.
Farrants says the project would not have been possible without the interaction and collaboration that happens at Janelia, which enables her and other chemists to build tools that biologists need and want.
“I really enjoy tinkering with things and building tools, but if I know that what I am building and tinkering with has an application that someone is going to care about, I think that is what makes it fun and rewarding,” Farrants says. “That’s what I like about Janelia: You get to interact with people on a daily basis. It happens in the wider scientific world as well, but Janelia is a special place.”
###
Citation:
Helen Farrants, Yichun Shuai, William C. Lemon, Christian Monroy Hernandez, Deng Zhang, Shang Yang, Ronak Patel, Guanda Qiao, Michelle S. Frei, Jonathan B. Grimm, Timothy L. Hanson, Filip Tomaska, Glenn C. Turner, Carsen Stringer, Philipp J. Keller, Abraham G. Beyene, Yao Chen, Yajie Liang, Luke D. Lavis, and Eric R. Schreiter. “A modular chemigenetic calcium indicator for multiplexed in vivo functional imaging.” Published September 20, 2024. DOI: 10.1038/s41592-024-02411-6
Media Contacts
Nanci Bompey












