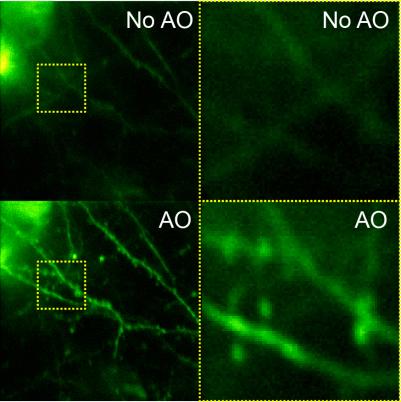
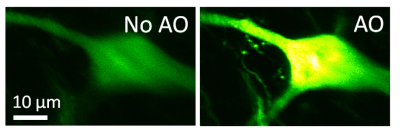
Imaging resolution in vivo is limited by the refractive index mismatch between biological samples and the media for which a microscope objective is designed. It is not possible to achieve diffraction-limited resolution without correcting for these sample-induced aberrations. Using mouse brain as our model system, we develop scattering-resistant adaptive optical methods to cancel out these aberrations and achieve high-resolution imaging in vivo.
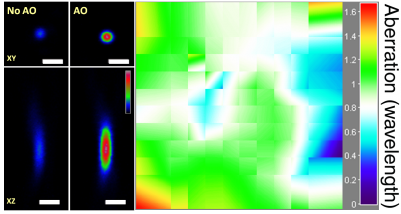
Ideal imaging occurs when we have a diffraction-limited focus, where all light rays intersect and interfere constructively. In one approach, we first direct rays to intersect at the same point by measuring image shifts when the corresponding segments of back pupil are illuminated, and then find the phase for those rays by either interference or phase reconstruction.
In an alternative approach, to avoid the numerical aperture (NA) reduction associated with single-segment illumination, we illuminate the full pupil and recover a perfect focus by scanning one ray around the focus formed by all other rays while monitoring the focal intensity variation.
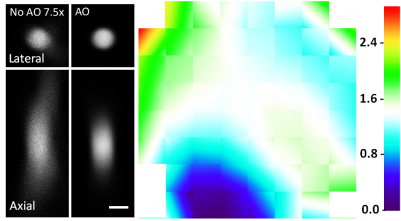
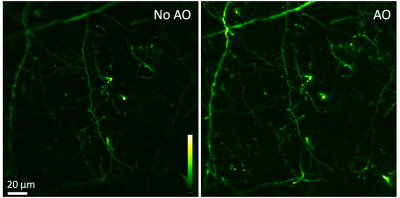
Speeding up the full-pupil method by multiplexing, we can rapidly measure aberration in scattering tissues and achieve corrections applicable over hundreds of microns inside the mouse brain.
By taking advantage of the reduced scattering of near-infrared light in the mouse brain, we also demonstrated that direct wavefront sensing can be applied at 700 μm depth.
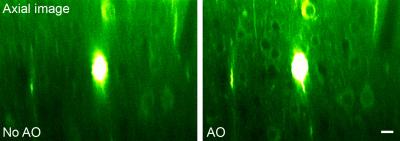
To image deep nuclei in vivo, microendoscopy offers a minimally invasive approach, with the most popular implementations employing gradient refractive-index (GRIN) lenses. Unlike conventional objective lenses where many optical elements are used to minimize off-axis aberration, high-NA GRIN lenses are plagued with intrinsic aberrations. By correcting these aberrations, we can increase their imaging performance in 3D.