Filter
Associated Lab
- Ahrens Lab (2) Apply Ahrens Lab filter
- Aso Lab (1) Apply Aso Lab filter
- Baker Lab (1) Apply Baker Lab filter
- Branson Lab (1) Apply Branson Lab filter
- Druckmann Lab (3) Apply Druckmann Lab filter
- Harris Lab (3) Apply Harris Lab filter
- Hermundstad Lab (9) Apply Hermundstad Lab filter
- Hess Lab (1) Apply Hess Lab filter
- Jayaraman Lab (46) Apply Jayaraman Lab filter
- Ji Lab (1) Apply Ji Lab filter
- Karpova Lab (1) Apply Karpova Lab filter
- Looger Lab (10) Apply Looger Lab filter
- Podgorski Lab (1) Apply Podgorski Lab filter
- Reiser Lab (2) Apply Reiser Lab filter
- Romani Lab (5) Apply Romani Lab filter
- Rubin Lab (6) Apply Rubin Lab filter
- Saalfeld Lab (1) Apply Saalfeld Lab filter
- Scheffer Lab (1) Apply Scheffer Lab filter
- Schreiter Lab (9) Apply Schreiter Lab filter
- Svoboda Lab (9) Apply Svoboda Lab filter
- Zlatic Lab (1) Apply Zlatic Lab filter
Associated Project Team
Publication Date
- 2025 (1) Apply 2025 filter
- 2024 (3) Apply 2024 filter
- 2023 (1) Apply 2023 filter
- 2022 (3) Apply 2022 filter
- 2021 (1) Apply 2021 filter
- 2020 (4) Apply 2020 filter
- 2019 (4) Apply 2019 filter
- 2018 (3) Apply 2018 filter
- 2017 (4) Apply 2017 filter
- 2016 (3) Apply 2016 filter
- 2015 (4) Apply 2015 filter
- 2014 (1) Apply 2014 filter
- 2013 (3) Apply 2013 filter
- 2012 (2) Apply 2012 filter
- 2011 (2) Apply 2011 filter
- 2010 (2) Apply 2010 filter
- 2009 (2) Apply 2009 filter
- 2007 (1) Apply 2007 filter
- 2006 (1) Apply 2006 filter
- 2003 (1) Apply 2003 filter
Type of Publication
46 Publications
Showing 11-20 of 46 resultsClock neurons generate circadian rhythms in behavioral activity, but the relevant pathways remain poorly understood. In this issue of Neuron, Liang et al. (2019) show that distinct clock neurons independently drive movement-promoting “ring neurons” in Drosophila through dopaminergic relays to support morning and evening locomotor activity.
View Publication PageAnimals use acoustic signals across a variety of social behaviors, particularly courtship. In Drosophila, song is detected by antennal mechanosensory neurons and further processed by second-order aPN1/aLN(al) neurons. However, little is known about the central pathways mediating courtship hearing. In this study, we identified a male-specific pathway for courtship hearing via third-order ventrolateral protocerebrum Projection Neuron 1 (vPN1) neurons and fourth-order pC1 neurons. Genetic inactivation of vPN1 or pC1 disrupts song-induced male-chaining behavior. Calcium imaging reveals that vPN1 responds preferentially to pulse song with long inter-pulse intervals (IPIs), while pC1 responses to pulse song closely match the behavioral chaining responses at different IPIs. Moreover, genetic activation of either vPN1 or pC1 induced courtship chaining, mimicking the behavioral response to song. These results outline the aPN1-vPN1-pC1 pathway as a labeled line for the processing and transformation of courtship song in males.
Seizures induced by visual stimulation (photosensitive epilepsy; PSE) represent a common type of epilepsy in humans, but the molecular mechanisms and genetic drivers underlying PSE remain unknown, and no good genetic animal models have been identified as yet. Here, we show an animal model of PSE, in , owing to defective cortex glia. The cortex glial membranes are severely compromised in ceramide phosphoethanolamine synthase ()-null mutants and fail to encapsulate the neuronal cell bodies in the neuronal cortex. Expression of human sphingomyelin synthase 1, which synthesizes the closely related ceramide phosphocholine (sphingomyelin), rescues the cortex glial abnormalities and PSE, underscoring the evolutionarily conserved role of these lipids in glial membranes. Further, we show the compromise in plasma membrane structure that underlies the glial cell membrane collapse in mutants and leads to the PSE phenotype.
Behavioral strategies employed for chemotaxis have been described across phyla, but the sensorimotor basis of this phenomenon has seldom been studied in naturalistic contexts. Here, we examine how signals experienced during free olfactory behaviors are processed by first-order olfactory sensory neurons (OSNs) of the Drosophila larva. We find that OSNs can act as differentiators that transiently normalize stimulus intensity-a property potentially derived from a combination of integral feedback and feed-forward regulation of olfactory transduction. In olfactory virtual reality experiments, we report that high activity levels of the OSN suppress turning, whereas low activity levels facilitate turning. Using a generalized linear model, we explain how peripheral encoding of olfactory stimuli modulates the probability of switching from a run to a turn. Our work clarifies the link between computations carried out at the sensory periphery and action selection underlying navigation in odor gradients.
Odors evoke complex responses in locust antennal lobe projection neurons (PNs)-the mitral cell analogs. These patterns evolve over hundreds of milliseconds and contain information about odor identity and concentration. In nature, animals often encounter many odorants in short temporal succession. We explored the effects of such conditions by presenting two different odors with variable intervening delays. PN ensemble representations tracked stimulus changes and, in some delay conditions, reached states that corresponded neither to the representation of either odor alone nor to the static mixture of the two. We then recorded from Kenyon cells (KCs), the PNs’ targets. Their responses were consistent with the PN population’s behavior: in some conditions, KCs were recruited that did not fire during single-odor or mixture stimuli. Thus, PN population dynamics are history dependent, and responses of individual KCs are consistent with piecewise temporal decoding of PN output over large sections of the PN population.
Genetically encoded optical indicators hold the promise of enabling non-invasive monitoring of activity in identified neurons in behaving organisms. However, the interpretation of images of brain activity produced using such sensors is not straightforward. Several recent studies of sensory coding used G-CaMP 1.3-a calcium sensor-as an indicator of neural activity; some of these studies characterized the imaged neurons as having narrow tuning curves, a conclusion not always supported by parallel electrophysiological studies. To better understand the possible cause of these conflicting results, we performed simultaneous in vivo 2-photon imaging and electrophysiological recording of G-CaMP 1.3 expressing neurons in the antennal lobe (AL) of intact fruitflies. We find that G-CaMP has a relatively high threshold, that its signal often fails to capture spiking response kinetics, and that it can miss even high instantaneous rates of activity if those are not sustained. While G-CaMP can be misleading, it is clearly useful for the identification of promising neural targets: when electrical activity is well above the sensor’s detection threshold, its signal is fairly well correlated with mean firing rate and G-CaMP does not appear to alter significantly the responses of neurons that express it. The methods we present should enable any genetically encoded sensor, activator, or silencer to be evaluated in an intact neural circuit in vivo in Drosophila.
After finding food, a foraging animal must decide whether to continue feeding, or to explore the environment for potentially better options. One strategy to negotiate this tradeoff is to perform local searches around the food but repeatedly return to feed. We studied this behavior in flies and used genetic tools to uncover the underlying mechanisms. Over time, flies gradually expand their search, shifting from primarily exploiting food sources to exploring the environment, a change that is likely driven by increases in satiety. We found that flies’ search patterns preserve these dynamics even as the overall scale of the search is modulated by starvation-induced changes in metabolic state. In contrast, search induced by optogenetic activation of sugar sensing neurons does not show these dynamics. We asked what navigational strategies underlie local search. Using a generative model, we found that a change in locomotor pattern after food consumption could account for repeated returns to the food, but failed to capture relatively direct, long return trajectories. Alternative strategies, such as path integration or sensory taxis could allow flies to return from larger distances. We tested this by individually silencing the fly’s head direction system, olfaction and hygrosensation, and found that the only substantial effect was from perturbing hygrosensation, which reduced the number of long exploratory trips. Our study illustrates that local search is composed of multiple behavioral features that evolve over time based on both internal and external factors, providing a path towards uncovering the underlying neural mechanisms.
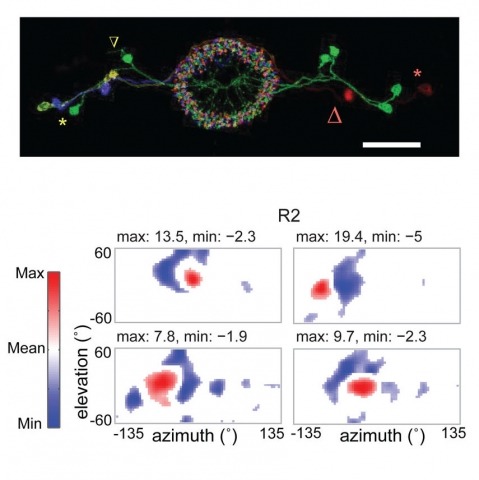
Many animals, including insects, are known to use visual landmarks to orient in their environment. In Drosophila melanogaster, behavioural genetics studies have identified a higher brain structure called the central complex as being required for the fly’s innate responses to vertical visual features and its short- and long-term memory for visual patterns. But whether and how neurons of the fly central complex represent visual features are unknown. Here we use two-photon calcium imaging in head-fixed walking and flying flies to probe visuomotor responses of ring neurons—a class of central complex neurons that have been implicated in landmark-driven spatial memory in walking flies and memory for visual patterns in tethered flying flies. We show that dendrites of ring neurons are visually responsive and arranged retinotopically. Ring neuron receptive fields comprise both excitatory and inhibitory subfields, resembling those of simple cells in the mammalian primary visual cortex. Ring neurons show strong and, in some cases, direction-selective orientation tuning, with a notable preference for vertically oriented features similar to those that evoke innate responses in flies. Visual responses were diminished during flight, but, in contrast with the hypothesized role of the central complex in the control of locomotion, not modulated during walking. Taken together, these results indicate that ring neurons represent behaviourally relevant visual features in the fly’s environment, enabling downstream central complex circuits to produce appropriate motor commands. More broadly, this study opens the door to mechanistic investigations of circuit computations underlying visually guided action selection in the Drosophila central complex.
Internal representations are thought to support the generation of flexible, long-timescale behavioral patterns in both animals and artificial agents. Here, we present a novel conceptual framework for how Drosophila use their internal representation of head direction to maintain preferred headings in their surroundings, and how they learn to modify these preferences in the presence of selective thermal reinforcement. To develop the framework, we analyzed flies’ behavior in a classical operant visual learning paradigm and found that they use stochastically generated fixations and directed turns to express their heading preferences. Symmetries in the visual scene used in the paradigm allowed us to expose how flies’ probabilistic behavior in this setting is tethered to their head direction representation. We describe how flies’ ability to quickly adapt their behavior to the rules of their environment may rest on a behavioral policy whose parameters are flexible but whose form is genetically encoded in the structure of their circuits. Many of the mechanisms we outline may also be relevant for rapidly adaptive behavior driven by internal representations in other animals, including mammals.