Filter
Associated Lab
- Ahrens Lab (2) Apply Ahrens Lab filter
- Aso Lab (1) Apply Aso Lab filter
- Baker Lab (1) Apply Baker Lab filter
- Branson Lab (1) Apply Branson Lab filter
- Druckmann Lab (3) Apply Druckmann Lab filter
- Harris Lab (3) Apply Harris Lab filter
- Hermundstad Lab (9) Apply Hermundstad Lab filter
- Hess Lab (1) Apply Hess Lab filter
- Jayaraman Lab (46) Apply Jayaraman Lab filter
- Ji Lab (1) Apply Ji Lab filter
- Karpova Lab (1) Apply Karpova Lab filter
- Looger Lab (10) Apply Looger Lab filter
- Podgorski Lab (1) Apply Podgorski Lab filter
- Reiser Lab (2) Apply Reiser Lab filter
- Romani Lab (5) Apply Romani Lab filter
- Rubin Lab (6) Apply Rubin Lab filter
- Saalfeld Lab (1) Apply Saalfeld Lab filter
- Scheffer Lab (1) Apply Scheffer Lab filter
- Schreiter Lab (9) Apply Schreiter Lab filter
- Svoboda Lab (9) Apply Svoboda Lab filter
- Zlatic Lab (1) Apply Zlatic Lab filter
Associated Project Team
Publication Date
- 2025 (1) Apply 2025 filter
- 2024 (3) Apply 2024 filter
- 2023 (1) Apply 2023 filter
- 2022 (3) Apply 2022 filter
- 2021 (1) Apply 2021 filter
- 2020 (4) Apply 2020 filter
- 2019 (4) Apply 2019 filter
- 2018 (3) Apply 2018 filter
- 2017 (4) Apply 2017 filter
- 2016 (3) Apply 2016 filter
- 2015 (4) Apply 2015 filter
- 2014 (1) Apply 2014 filter
- 2013 (3) Apply 2013 filter
- 2012 (2) Apply 2012 filter
- 2011 (2) Apply 2011 filter
- 2010 (2) Apply 2010 filter
- 2009 (2) Apply 2009 filter
- 2007 (1) Apply 2007 filter
- 2006 (1) Apply 2006 filter
- 2003 (1) Apply 2003 filter
Type of Publication
46 Publications
Showing 21-30 of 46 resultsMany animals rely on an internal heading representation when navigating in varied environments. How this representation is linked to the sensory cues that define different surroundings is unclear. In the fly brain, heading is represented by 'compass' neurons that innervate a ring-shaped structure known as the ellipsoid body. Each compass neuron receives inputs from 'ring' neurons that are selective for particular visual features; this combination provides an ideal substrate for the extraction of directional information from a visual scene. Here we combine two-photon calcium imaging and optogenetics in tethered flying flies with circuit modelling, and show how the correlated activity of compass and visual neurons drives plasticity, which flexibly transforms two-dimensional visual cues into a stable heading representation. We also describe how this plasticity enables the fly to convert a partial heading representation, established from orienting within part of a novel setting, into a complete heading representation. Our results provide mechanistic insight into the memory-related computations that are essential for flexible navigation in varied surroundings.
Calcium imaging with genetically encoded calcium indicators (GECIs) is routinely used to measure neural activity in intact nervous systems. GECIs are frequently used in one of two different modes: to track activity in large populations of neuronal cell bodies, or to follow dynamics in subcellular compartments such as axons, dendrites and individual synaptic compartments. Despite major advances, calcium imaging is still limited by the biophysical properties of existing GECIs, including affinity, signal-to-noise ratio, rise and decay kinetics and dynamic range. Using structure-guided mutagenesis and neuron-based screening, we optimized the green fluorescent protein-based GECI GCaMP6 for different modes of in vivo imaging. The resulting jGCaMP7 sensors provide improved detection of individual spikes (jGCaMP7s,f), imaging in neurites and neuropil (jGCaMP7b), and may allow tracking larger populations of neurons using two-photon (jGCaMP7s,f) or wide-field (jGCaMP7c) imaging.
Calcium imaging with genetically encoded calcium indicators (GECIs) is routinely used to measure neural activity in intact nervous systems. GECIs are frequently used in one of two different modes: to track activity in large populations of neuronal cell bodies, or to follow dynamics in subcellular compartments such as axons, dendrites and individual synaptic compartments. Despite major advances, calcium imaging is still limited by the biophysical properties of existing GECIs, including affinity, signal-to-noise ratio, rise and decay kinetics, and dynamic range. Using structure-guided mutagenesis and neuron-based screening, we optimized the green fluorescent protein-based GECI GCaMP6 for different modes of in vivo imaging. The jGCaMP7 sensors provide improved detection of individual spikes (jGCaMP7s,f), imaging in neurites and neuropil (jGCaMP7b), and tracking large populations of neurons using 2-photon (jGCaMP7s,f) or wide-field (jGCaMP7c) imaging.
Genetically encoded calcium indicators (GECIs) can be used to image activity in defined neuronal populations. However, current GECIs produce inferior signals compared to synthetic indicators and recording electrodes, precluding detection of low firing rates. We developed a single-wavelength GCaMP2-based GECI (GCaMP3), with increased baseline fluorescence (3-fold), increased dynamic range (3-fold) and higher affinity for calcium (1.3-fold). We detected GCaMP3 fluorescence changes triggered by single action potentials in pyramidal cell dendrites, with signal-to-noise ratio and photostability substantially better than those of GCaMP2, D3cpVenus and TN-XXL. In Caenorhabditis elegans chemosensory neurons and the Drosophila melanogaster antennal lobe, sensory stimulation-evoked fluorescence responses were significantly enhanced with GCaMP3 (4-6-fold). In somatosensory and motor cortical neurons in the intact mouse, GCaMP3 detected calcium transients with amplitudes linearly dependent on action potential number. Long-term imaging in the motor cortex of behaving mice revealed large fluorescence changes in imaged neurons over months.
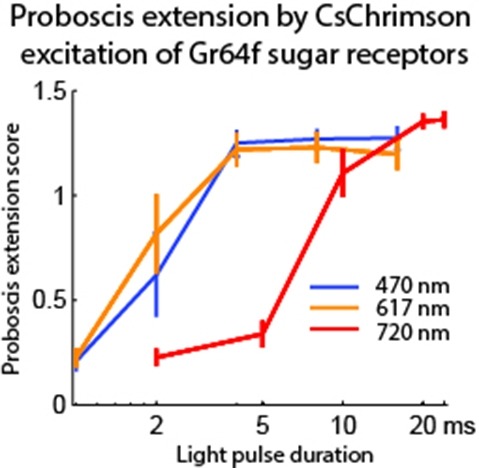
Optogenetic tools enable examination of how specific cell types contribute to brain circuit functions. A long-standing question is whether it is possible to independently activate two distinct neural populations in mammalian brain tissue. Such a capability would enable the study of how different synapses or pathways interact to encode information in the brain. Here we describe two channelrhodopsins, Chronos and Chrimson, discovered through sequencing and physiological characterization of opsins from over 100 species of alga. Chrimson’s excitation spectrum is red shifted by 45 nm relative to previous channelrhodopsins and can enable experiments in which red light is preferred. We show minimal visual system-mediated behavioral interference when using Chrimson in neurobehavioral studies in Drosophila melanogaster. Chronos has faster kinetics than previous channelrhodopsins yet is effectively more light sensitive. Together these two reagents enable two-color activation of neural spiking and downstream synaptic transmission in independent neural populations without detectable cross-talk in mouse brain slice.
We examined the encoding and decoding of odor identity and intensity by neurons in the antennal lobe and the mushroom body, first and second relays, respectively, of the locust olfactory system. Increased odor concentration led to changes in the firing patterns of individual antennal lobe projection neurons (PNs), similar to those caused by changes in odor identity, thus potentially confounding representations for identity and concentration. However, when these time-varying responses were examined across many PNs, concentration-specific patterns clustered by identity, resolving the apparent confound. This is because PN ensemble representations changed relatively continuously over a range of concentrations of each odorant. The PNs’ targets in the mushroom body-Kenyon cells (KCs)-had sparse identity-specific responses with diverse degrees of concentration invariance. The tuning of KCs to identity and concentration and the patterning of their responses are consistent with piecewise decoding of their PN inputs over oscillation-cycle length epochs.
The identification of active neurons and circuits in vivo is a fundamental challenge in understanding the neural basis of behavior. Genetically encoded calcium (Ca(2+)) indicators (GECIs) enable quantitative monitoring of cellular-resolution activity during behavior. However, such indicators require online monitoring within a limited field of view. Alternatively, post hoc staining of immediate early genes (IEGs) indicates highly active cells within the entire brain, albeit with poor temporal resolution. We designed a fluorescent sensor, CaMPARI, that combines the genetic targetability and quantitative link to neural activity of GECIs with the permanent, large-scale labeling of IEGs, allowing a temporally precise "activity snapshot" of a large tissue volume. CaMPARI undergoes efficient and irreversible green-to-red conversion only when elevated intracellular Ca(2+) and experimenter-controlled illumination coincide. We demonstrate the utility of CaMPARI in freely moving larvae of zebrafish and flies, and in head-fixed mice and adult flies.
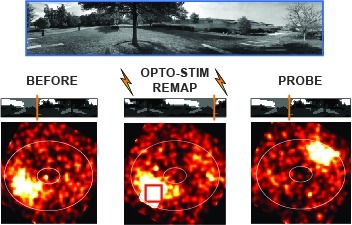
Many animals rely on a representation of head direction for flexible, goal-directed navigation. In insects, a compass-like head direction representation is maintained in a conserved brain region called the central complex. This head direction representation is updated by self-motion information and by tethering to sensory cues in the surroundings through a plasticity mechanism. However, under natural settings, some of these sensory cues may temporarily disappear—for example, when clouds hide the sun—and prominent landmarks at different distances from the insect may move across the animal's field of view during translation, creating potential conflicts for a neural compass. We used two-photon calcium imaging in head-fixed Drosophila behaving in virtual reality to monitor the fly's compass during navigation in immersive naturalistic environments with approachable local landmarks. We found that the fly's compass remains stable even in these settings by tethering to available global cues, likely preserving the animal's ability to perform compass-driven behaviors such as maintaining a constant heading.
Many animals rely on persistent internal representations of continuous variables for working memory, navigation, and motor control. Existing theories typically assume that large networks of neurons are required to maintain such representations accurately; networks with few neurons are thought to generate discrete representations. However, analysis of two-photon calcium imaging data from tethered flies walking in darkness suggests that their small head-direction system can maintain a surprisingly continuous and accurate representation. We thus ask whether it is possible for a small network to generate a continuous, rather than discrete, representation of such a variable. We show analytically that even very small networks can be tuned to maintain continuous internal representations, but this comes at the cost of sensitivity to noise and variations in tuning. This work expands the computational repertoire of small networks, and raises the possibility that larger networks could represent more and higher-dimensional variables than previously thought.
Many animals use an internal sense of direction to guide their movements through the world. Neurons selective to head direction are thought to support this directional sense and have been found in a diverse range of species, from insects to primates, highlighting their evolutionary importance. Across species, most head-direction networks share four key properties: a unique representation of direction at all times, persistent activity in the absence of movement, integration of angular velocity to update the representation, and the use of directional cues to correct drift. The dynamics of theorized network structures called ring attractors elegantly account for these properties, but their relationship to brain circuits is unclear. Here, we review experiments in rodents and flies that offer insights into potential neural implementations of ring attractor networks. We suggest that a theory-guided search across model systems for biological mechanisms that enable such dynamics would uncover general principles underlying head-direction circuit function. Expected final online publication date for the , Volume 43 is July 8, 2020. Please see http://www.annualreviews.org/page/journal/pubdates for revised estimates.