Filter
Associated Lab
- Aso Lab (1) Apply Aso Lab filter
- Branson Lab (1) Apply Branson Lab filter
- Card Lab (4) Apply Card Lab filter
- Cardona Lab (17) Apply Cardona Lab filter
- Dickson Lab (1) Apply Dickson Lab filter
- Fetter Lab (9) Apply Fetter Lab filter
- Heberlein Lab (1) Apply Heberlein Lab filter
- Riddiford Lab (17) Apply Riddiford Lab filter
- Rubin Lab (4) Apply Rubin Lab filter
- Simpson Lab (2) Apply Simpson Lab filter
- Singer Lab (1) Apply Singer Lab filter
- Stern Lab (4) Apply Stern Lab filter
- Truman Lab (88) Apply Truman Lab filter
- Zlatic Lab (13) Apply Zlatic Lab filter
Associated Project Team
Publication Date
- 2023 (2) Apply 2023 filter
- 2021 (3) Apply 2021 filter
- 2020 (3) Apply 2020 filter
- 2019 (4) Apply 2019 filter
- 2018 (8) Apply 2018 filter
- 2017 (6) Apply 2017 filter
- 2016 (11) Apply 2016 filter
- 2015 (6) Apply 2015 filter
- 2014 (1) Apply 2014 filter
- 2013 (3) Apply 2013 filter
- 2012 (3) Apply 2012 filter
- 2011 (1) Apply 2011 filter
- 2010 (4) Apply 2010 filter
- 2009 (4) Apply 2009 filter
- 2008 (2) Apply 2008 filter
- 2007 (1) Apply 2007 filter
- 2006 (3) Apply 2006 filter
- 2005 (2) Apply 2005 filter
- 2004 (2) Apply 2004 filter
- 2003 (1) Apply 2003 filter
- 2002 (1) Apply 2002 filter
- 2001 (2) Apply 2001 filter
- 2000 (2) Apply 2000 filter
- 1999 (1) Apply 1999 filter
- 1998 (2) Apply 1998 filter
- 1997 (2) Apply 1997 filter
- 1996 (1) Apply 1996 filter
- 1994 (1) Apply 1994 filter
- 1991 (1) Apply 1991 filter
- 1990 (1) Apply 1990 filter
- 1987 (1) Apply 1987 filter
- 1986 (1) Apply 1986 filter
- 1976 (1) Apply 1976 filter
- 1970 (1) Apply 1970 filter
Type of Publication
88 Publications
Showing 81-88 of 88 resultsThe steroid hormone 20-hydroxyecdysone (20E) initiates metamorphosis in insects by signaling through the ecdysone receptor complex, a heterodimer of the ecdysone receptor (EcR) and ultraspiracle (USP). Analysis of usp mutant clones in the wing disc of Drosophila shows that in the absence of USP, early hormone responsive genes such as EcR, DHR3 and E75B fail to up-regulate in response to 20E, but other genes that are normally expressed later, such as (&bgr;)-Ftz-F1 and the Z1 isoform of the Broad-Complex (BRC-Z1), are expressed precociously. Sensory neuron formation and axonal outgrowth, two early metamorphic events, also occur prematurely. In vitro experiments with cultured wing discs showed that BRC-Z1 expression and early metamorphic development are rendered steroid-independent in the usp mutant clones. These results are consistent with a model in which these latter processes are induced by a signal arising during the middle of the last larval stage but suppressed by the unliganded EcR/USP complex. Our observations suggest that silencing by the unliganded EcR/USP receptor and the subsequent release of silencing by moderate steroid levels may play an important role in coordinating early phases of steroid driven development.
The sense of smell enables animals to react to long-distance cues according to learned and innate valences. Here, we have mapped with electron microscopy the complete wiring diagram of the Drosophila larval antennal lobe, an olfactory neuropil similar to the vertebrate olfactory bulb. We found a canonical circuit with uniglomerular projection neurons (uPNs) relaying gain-controlled ORN activity to the mushroom body and the lateral horn. A second, parallel circuit with multiglomerular projection neurons (mPNs) and hierarchically connected local neurons (LNs) selectively integrates multiple ORN signals already at the first synapse. LN-LN synaptic connections putatively implement a bistable gain control mechanism that either computes odor saliency through panglomerular inhibition, or allows some glomeruli to respond to faint aversive odors in the presence of strong appetitive odors. This complete wiring diagram will support experimental and theoretical studies towards bridging the gap between circuits and behavior.
A plexus of multidendritic sensory neurons, the dendritic arborization (da) neurons, innervates the epidermis of soft-bodied insects. Previous studies have indicated that the plexus may comprise distinct subtypes of da neurons, which utilize diverse cyclic 3’,5’-guanosine monophosphate signaling pathways and could serve several functions. Here, we identify three distinct classes of da neurons in Manduca, which we term the alpha, beta, and gamma classes. These three classes differ in their sensory responses, branch complexity, peripheral dendritic fields, and axonal projections. The two identified alpha neurons branch over defined regions of the body wall, which in some cases correspond to specific natural folds of the cuticle. These cells project to an intermediate region of the neuropil and appear to function as proprioceptors. Three beta neurons are characterized by long, sinuous dendritic branches and axons that terminate in the ventral neuropil. The function of this group of neurons is unknown. Four neurons belonging to the gamma class have the most complex peripheral dendrites. A representative gamma neuron responds to forceful touch of the cuticle. Although the dendrites of da neurons of different classes may overlap extensively, cells belonging to the same class show minimal dendritic overlap. As a result, the body wall is independently tiled by the beta and gamma da neurons and partially innervated by the alpha neurons. These properties of the da system likely allow insects to discriminate the quality and location of several types of stimuli acting on the cuticle.
Higher-order genome organization plays an important role in transcriptional regulation. In Drosophila, somatic pairing of homologous chromosomes can lead to transvection, by which the regulatory region of a gene can influence transcription in trans. We observe transvection between transgenes inserted at commonly used phiC31 integration sites in the Drosophila genome. When two transgenes that carry endogenous regulatory elements driving the expression of either LexA or GAL4 are inserted at the same integration site and paired, the enhancer of one transgene can drive or repress expression of the paired transgene. These transvection effects depend on compatibility between regulatory elements and are often restricted to a subset of cell types within a given expression pattern. We further show that activated UAS-transgenes can also drive transcription in trans. We discuss the implication of these findings for 1) understanding the molecular mechanisms that underlie transvection and 2) the design of experiments that utilize site-specific integration.
BACKGROUND: In holometabolous insects such as Drosophila melanogaster, neuroblasts produce an initial population of diverse neurons during embryogenesis and a much larger set of adult-specific neurons during larval life. In the ventral CNS, many of these secondary neuronal lineages differ significantly from one body segment to another, suggesting a role for anteroposterior patterning genes. RESULTS: Here we systematically characterize the expression pattern and function of the Hox gene Ultrabithorax (Ubx) in all 25 postembryonic lineages. We find that Ubx is expressed in a segment-, lineage-, and hemilineage-specific manner in the thoracic and anterior abdominal segments. When Ubx is removed from neuroblasts via mitotic recombination, neurons in these segments exhibit the morphologies and survival patterns of their anterior thoracic counterparts. Conversely, when Ubx is ectopically expressed in anterior thoracic segments, neurons exhibit complementary posterior transformation phenotypes. CONCLUSION: Our findings demonstrate that Ubx plays a critical role in conferring segment-appropriate morphology and survival on individual neurons in the adult-specific ventral CNS. Moreover, while always conferring spatial identity in some sense, Ubx has been co-opted during evolution for distinct and even opposite functions in different neuronal hemilineages.
Neuroendocrine systems in animals maintain organismal homeostasis and regulate stress response. Although a great deal of work has been done on the neuropeptides and hormones that are released and act on target organs in the periphery, the synaptic inputs onto these neuroendocrine outputs in the brain are less well understood. Here, we use the transmission electron microscopy reconstruction of a whole central nervous system in the larva to elucidate the sensory pathways and the interneurons that provide synaptic input to the neurosecretory cells projecting to the endocrine organs. Predicted by network modeling, we also identify a new carbon dioxide-responsive network that acts on a specific set of neurosecretory cells and that includes those expressing corazonin (Crz) and diuretic hormone 44 (Dh44) neuropeptides. Our analysis reveals a neuronal network architecture for combinatorial action based on sensory and interneuronal pathways that converge onto distinct combinations of neuroendocrine outputs.
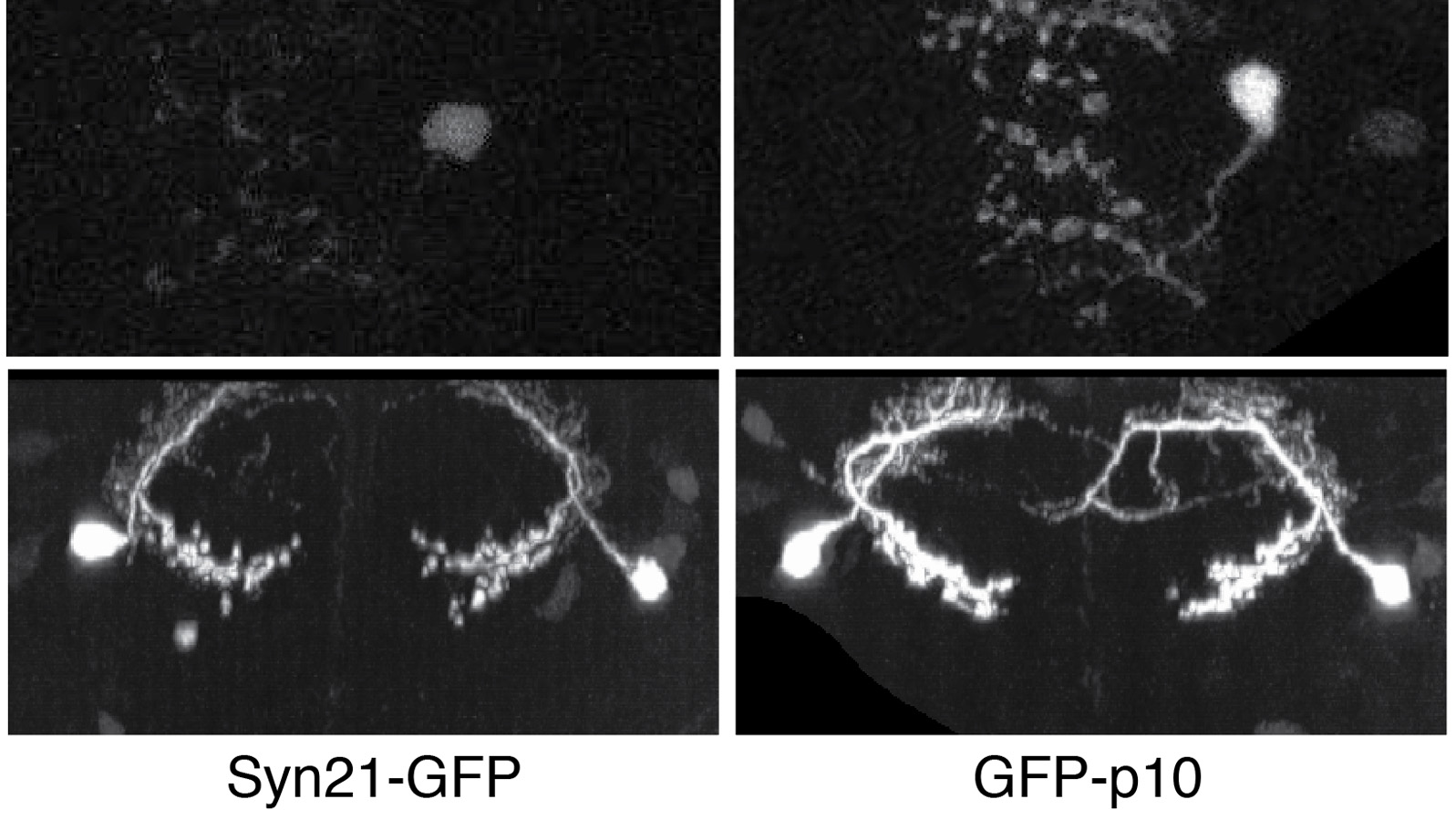
The ability to specify the expression levels of exogenous genes inserted in the genomes of transgenic animals is critical for the success of a wide variety of experimental manipulations. Protein production can be regulated at the level of transcription, mRNA transport, mRNA half-life, or translation efficiency. In this report, we show that several well-characterized sequence elements derived from plant and insect viruses are able to function in Drosophila to increase the apparent translational efficiency of mRNAs by as much as 20-fold. These increases render expression levels sufficient for genetic constructs previously requiring multiple copies to be effective in single copy, including constructs expressing the temperature-sensitive inactivator of neuronal function Shibire(ts1), and for the use of cytoplasmic GFP to image the fine processes of neurons.