Project objectives
(1) To create a collection of 200-400 “Generation 2” GAL4 driver lines, each with an expression pattern restricted to a single type of descending interneuron. We are targeting interneurons that have dendritic arbors in the brain and axonal projections to wing or leg neuropil of the ventral nerve cord.
(2) To use these lines to assay the functional role of descending interneurons in walking and flying behaviors. We will use a combination of cell silencing and cell activation techniques to explore the function of specific cells in both tethered and freely-moving flies.
Background
As flies traverse complex environments, they continuously integrate sensory information and send motor commands that influence complex behaviors. These signals pass from the fly’s brain to motor areas in the Ventral Nerve Cord (VNC) via the fly's neck, a bottleneck in the sensory-motor circuits that mediate many behaviors. Targeted manipulations of descending neuron activity may expose interpretable behavioral phenotypes that circuit redundancies up or downstream can mask. Indeed, work in other insect species suggests that this population of cells includes “command interneurons” that are capable of triggering complete motor actions.
Between 3,000 and 4,000 axons pass through the fly's neck, and we expect that about 700 of these are descending interneurons. Since the nervous system is bilaterally symmetrical, our best guess is that there are about 350 distinct pairs of descending cells.
We are collaborating with the FlyLight project team to make and image the driver lines. We, and our collaborators, are using these driver lines to assay the behavioral consequences of activating DNs in sensory guided behavioral circuits.
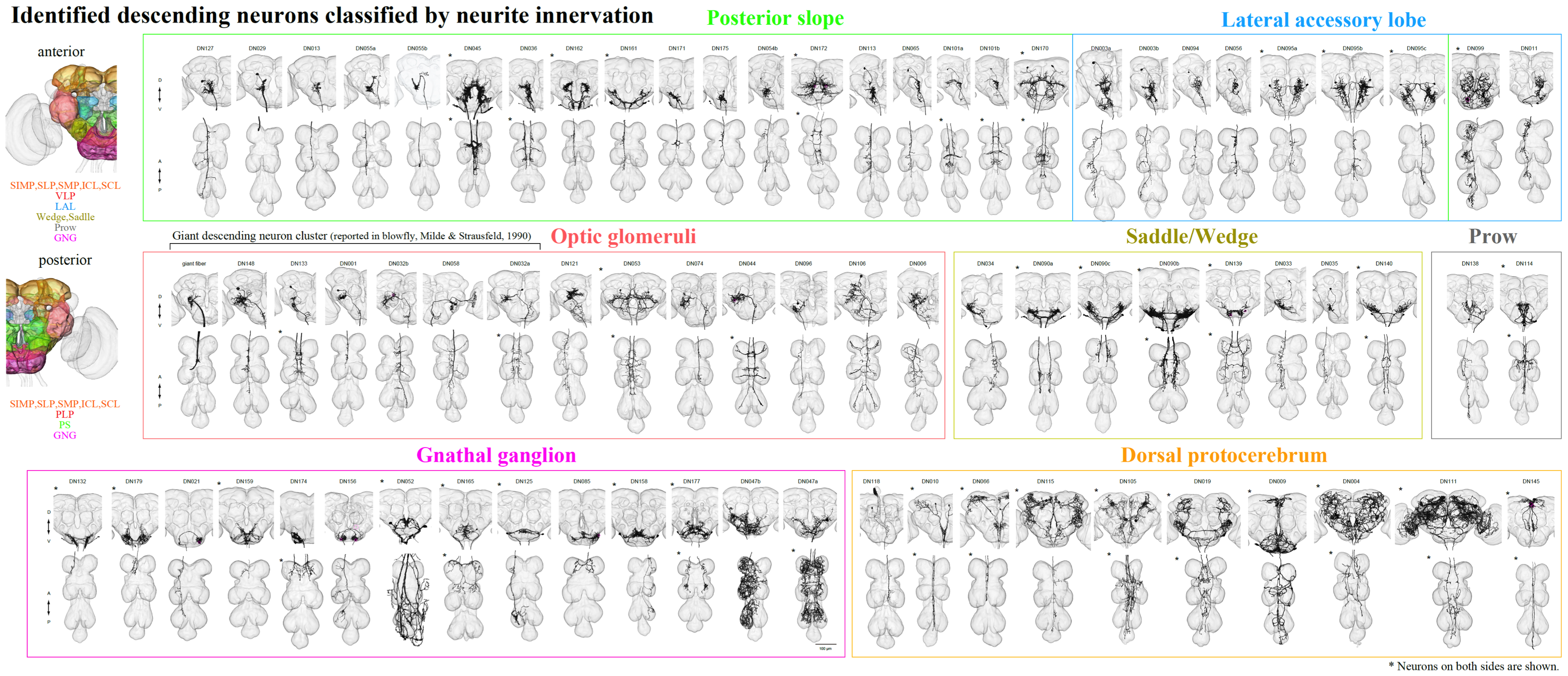
Progress
By screening the Janelia and Vienna Tiles (VT) GAL4 collections, we have manually segmented 146 Descending Interneurons (DNs) out of an estimated 300-500 DNs. Of these, we believe 89 are unique cell types and we have identified 74 DN not previously described in Drosophila.
This discovery phase was often aided by crossing to a GAL4 line expressing GAL80 under the control of the teashirt gene. The teashirt gene is expressed in the VNC and GAL80 blocks the function of GAL4. Thus in the F1 progeny, expression of GAL4-driven expression should be suppressed in ascending cells, whose cell bodies are in the TAG, but should not be affected in the axons of cells whose cell bodies are in the central brain. With the aid of FlyLight, we imaged 586 Gen1 GAL4 lines and discovered 23 new DN types using this approach. We have also identified and constructed 146 new split half lines for 45 unique DN cells that weren’t available in either the VT or Janelia split-GAL4 collections.
We have now designed and attempted an initial split screen of 1,000 split-GAL4 intersections to target very small numbers of DNs. From this screen, approximately 12.5% yielded intersections that were deemed worthy of stabilizing. We currently have 15 lines that have been made homozygous that are in the FlyLight queue for Polarity and Multicolor Flip-Out characterization. In addition, we have about 100 lines that are being stabilized that cover 57 different DN types and will enter the FlyLight pipeline when ready.
We will continue to refine our selection of split halves and screen an estimated additional 1,000 additional initial splits and choose an estimated 125-175 of these to stabilize and image through the polarity and MCFO pipelines over the coming year.
