Accomplishments and Progress
- The Larval Olympiad Project was started in earnest on September 1st, 2011.
- Jim Truman’s laboratory has characterized more than 7,000 GAL4 lines for nervous system expression using a UAS-GFP in the Drosophila L3 larva as part of the FlyLight project. From this data set, Dr. Truman has identified ~1,000 GAL4 lines that will be used as drivers for inactivation and activation screens in the Larval Olympiad.
- Prior to the project launch, the basic assay platforms were developed in Dr. Zlatic’s laboratory. Tomoko Oyama in the Zlatic lab has so far tested 600 GAL4 lines in the vibration assay and found that 6% were significantly different in their head casting and turning in response to vibration (while the baseline turning rate was normal). As larvae were monitored before delivering a stimulus, lines that were significantly different from controls during their baseline (pre-stimulus) behavior were also detected. For example, 12% of the lines were significantly different from controls in their baseline frequency of peristaltic waves.
- Tomoko Oyama in the Zlatic lab has tested ~1000 lines using UAS-channel rhodopsin. This screen revealed subsets of neurons whose activation was sufficient to induce specific motor patterns, without affecting other motor patterns. In addition, a number of lines were found whose activation was sufficient to induce specific sequences or combinations of two or more motor patterns. Some of these sequences were similar to sequences normally evoked by sensory stimuli. In some cases neurons that were found to be required for a specific motor pattern in the inactivation screens were also found to be sufficient to induce this motor pattern in the activation screen.
- An automated data analysis pipeline has been developed which includes automatic worm tracking and distills larval tracks into meaningful quantitative measurements. Detailed statistical analysis approaches for hit detection compared to wild-type behavior and functional correlations are being developed in collaboration with the Johns Hopkins University Department of Applied Mathematics and Statistics.
Behavioral Assays
Somatosensation and basic motor patterns
The larval somatosensory system is the most pronounced sensory system of the animal, with 44 neurons in each hemisegment (88 per segment and approximately 1000 cells in total). The somatosensory system comprises several different morphological classes: class I, II, III, and IV multidendritic (md-da) neurons, bipolar dendrite neurons (bd), external sensory organ neurons (es) and chordotonal neurons (ch). The functions of some of these neurons are partially understood. Class IV md neurons have previously been implicated in sensing noxious stimuli (noxious heat, noxious mechanical stimuli and noxious light) and mediating escape and avoidance responses (Hwang et al., 2007; Xiang et al., 2010). Ch neurons have been implicated in sensing mechanical stimuli, touch and stretch (Caldwell et al., 2003). The Zlatic lab has found ch neurons also mediate larval startle and avoidance reactions to vibration and sound (Wu et al., in press; Ohyama, Zlatic, unpublished). Class I md neurons and the bd neurons have been implicated in sensing stretch and providing feedback during locomotion (Cheng et al., 2010; Hughes and Thomas, 2007; Song et al., 2007).
The Zlatic lab has developed quantitative, high-resolution and high-throughput assays for most larval motor patterns (targeting the circuitry downstream of proprioceptive neurons and motor pattern generating circuitry) for larval reactions: to noxious stimuli (targeting the circuitry downstream of Class IV neurons), to vibration and sound (targeting the circuitry downstream of ch neurons), to air puffs (responsible sensory neurons are unknown, likely es) and to the tactile stimuli, holes and grooves (responsible sensory neurons are unknown, candidates include es, md, ch, or all three).
These assays allow detailed characterization of the baseline behavior of wild type Drosophila larvae and of their reactions to each of these stimuli. Prior to most stimulus presentations, larvae are tracked for at least 30 seconds. During this period larvae are engaged in free foraging behavior, which consists of the basic locomotion due to peristaltic waves occasionally interrupted by head casting and turning. Interestingly, somatosensory stimuli then evoke, not single motor patterns, but stimulus-specific sequences and combinations of distinct motor patterns (Zlatic et al, unpublished). Vibration induces a startle/avoidance sequence: (1) head-retraction; (2) head-casting and turning; and (3) crawling forward at medium speed. Pain induces an escape/avoidance sequence: (1) curling; (2) rolling; and (3) crawling forward at high speed. Air puff induces a lot of head casting, turning and backward crawling followed by slow forward crawling. Holes induce digging, whereas grooves induce following (thigmotaxis).
These assays and the detailed description of the wild type behavior offer an excellent opportunity to identify neurons required for sensory processing and sensory-motor transformations as well as those required for the generation of each motor pattern and for stringing the motor patterns into appropriate sequences.
Activation screens using Channel Rhodopsin and TrpA1 will complement the inactivation screens and identify interneurons whose activation is sufficient to induce specific motor patterns or sequences of motor patterns.
Temperature and thermotaxis
The Larval Olympiad Project will bring the post doctoral visiting scientist Bruno Afonso from Aravi Samuel’s lab to perform an inactivation screen to identify neurons required for the processing of hot and cold stimuli and for larval thermotaxis.
The Samuel laboratory has developed quantitative high-resolution and high-throughput behavioral assays for larval navigation in thermal, light and olfactory gradients and has uncovered the basic strategies for thermotactic and phototactic navigation (Luo et al.). They find that larvae make discrete decisions during navigation by periodically interrupting forward movement to sweep their heads back and forth gathering sensory input. Following the head-casting events, larvae pick a new direction for forward crawling. The larvae modulate the frequency, size, and direction of these turning decisions to augment the likelihood that they point towards favorable environments at all times.
The primary sensory cells that are utilized for thermotaxis are known (Liu et al., 2003). This screen will confirm previous work, reveal interneurons downstream of the known sensory neurons required for the processing of thermal stimuli, and identify neurons in the central brain involved in encoding and implementing the behavioral strategy for thermotaxis.
Specifically, the assays involve subjecting each group of animals to several varying temperature gradients and tracking the behavior of animals. Statistical comparisons of individual larval movements during the different phases of the sensory input (e.g., warming vs. cooling) will be performed on the tracked data. In particular, the frequency, size, and direction of head sweeps, as well as the speed and straightness of periods of forward movement will be quantified.
The hardware for presentation of the thermal gradients to populations of larvae has been fully developed in the Samuel lab and the gradient control is fully automated (Luo et al.). Likewise, the software modules for detecting head casting, turning and crawling already exist and are part of the pipeline developed by the Zlatic lab for the automated detection of aberrant behaviors. Hence, all the data from this screen can be processed in exactly the same way as the data from the somatosensory screens.
Light and phototaxis
The Larval Olympiad Project will bring the postdoctoral visiting scientist Naryttza Diaz from Simon Sprecher’s lab to work together with Bruno Afonso from Aravi Samuel’s lab, and they will perform an inactivation screen to identify neurons required for the processing of light stimuli and for larval phototaxis.
Larvae display a strong photo-negative response when exposed to light. Interestingly this response is lost during the late larval stages (Sawin-McCormack et al., 1995). It is not known how the visual circuit acts to alter navigational logic. The Riddiford group is taking advantage of findings in other larvae (Dominick and Truman, 1985) that the premetamorphic shifts in behavior, like that seen for phototaxis, are due to the metamorphic hormone, ecdysone. They will be using the Rubin GAL4 collection to block ecdysone action in specific neurons, using a dominant-negative form of the ecdysone receptor (Cherbas et al., 2003), and then to test late-stage larvae for the loss of negative phototaxis.
Previous work by Sprecher et al. has found that larvae have eight green-sensitive (expressing Rhodopsin 6) and four blue-sensitive (expressing Rhodopsin 5) photoreceptors in each eye. All photoreceptors project their axons into the larval optic neuropile (LON) (Sprecher et al., 2007). Work from the Sprecher lab has identified about 12 interneurons connecting to the LON (Sprecher, Hartenstein, Cardona, unpublished), including direct postsynaptic targets of the photoreceptors as well as presynaptic neurons transmitting information from other brain centers into the LON (Diaz, Gendre, Sprecher, unpublished).
The knowledge of how the visual circuit controls behavior is still very limited. Higher brain centers for visual processing have not yet been identified. The phototaxis screen will identify central brain neurons, which confer specific aspects of visual information processing. Since the Sprecher lab has identified the “second order” neurons in visual processing, the identified higher order neurons will immediately be placed downstream of a defined photoreceptor-type and second order neuron, thus continuously adding novel components into the functional circuit.
The Sprecher and Samuel labs have established behavioral paradigms for studying visual behavior. Directional or temporal light-gradients can be used to stimulate phototaxis behavior. The rig for automated presentation of light gradients have already been developed in the Samuel and Sprecher labs. The changes in the behavioral sub-routines (peristalsis and head casting and turning) evoked by the light gradients will be analyzed in an automated way using the Multi Worm Tracking software and the Zlatic lab pipeline.
Odor and chemotaxis
A visiting scientist from Matthieu Louis’ lab will perform an inactivation screen together with Bruno Afonso from Aravi Samuel’s lab to identify neurons involved in the processing of odors and in larval chemotaxis.
Chemotaxis involves directed navigation toward attractive stimuli and away from aversive stimuli. This orientation process relies on the detection of small changes in odor concentrations. The larval olfactory system is the best characterized sensory system in Drosophila. The primary olfactory sensory neurons come from two bilateral olfactory organs called dorsal organs and project to the antennal lobe glomeruli where they form connections with local interneurons and projection neurons (Stocker, 2009). The second order projection neurons relay olfactory information to the mushroom body, implicated in associative memory formation, and to the lateral horn, implicated in hard-wired odor responses (Gerber et al., 2009; Masuda-Nakagawa et al., 2010; Masuda-Nakagawa et al., 2009; Scherer et al., 2003). However, how and where the olfactory information is linked to the motor output is not known.
Previous work by Louis et al. has shown that chemotaxis does not require comparisons between the left and right dorsal organs (Louis et al., 2008a). Instead, larvae ‘register’ their environment by translating their head on each side of the body axis. During lateral head sweeps, concentrations are sequentially sampled at different points in space. The Louis lab has found that executing a turn involves more than a binary decision: in addition to computing whether the concentration of an attractive stimulus is higher on the left or the right side, larvae modulate the amplitude of their turns to maximize their alignment orientation with the local odorant gradient.
The Louis lab now proposes to identify new central circuits controlling odor-driven orientation responses. They hypothesize that these circuits include a short-term memory coupled with a decision-making module. In adults, the central complex has been associated with similar goal-driven orientation tasks. They suggest that this screen may identify the larval equivalent(s) of the adult central complex.
The Louis lab has developed an assay for high-resolution measurement of odor-driven behavior (Louis et al., 2008b). The hardware module for automated presentation of odor gradients to populations of larvae has been developed in the Samuel lab and a version of this setup will be replicated at Janelia. The animals will be tracked with the Multi Worm Tracker software and data from this rig will be automatically processed by the Zlatic lab pipeline, as described before.
Feeding behavior
Dr. Feng Li of the Truman group has developed a multistage screen for identifying neurons that are involved in feeding behavior. The initial screen is a tonic suppression screen in which the GAL4 lines are crossed to a UAS-tetanus toxin effector. Larval lines showing normal growth rates are discarded but those that grow slowly or fail to grow altogether are then tested in a phasic screen. For the latter, the lines are crossed to UAS-shibirets and raised at 25C until the early 3rd larval stage. Larvae are removed from food, starved for 30 minutes at 25C and placed at restricted temperature of 32~33C for 5 minutes. The larvae then are left on a bed of colored yeast for 2~3 minutes allowing them to feed at 32~33C (the restrictive temperature). Larvae that show normal locomotion but do not have the colored yeast in their gut are candidate feeding hits. Validation of the approach is shown by the result that the silencing of the motoneurons that control the cibarial pump results in a lack of ingestion. The screen has already yielded candidate sensory neurons as well as interneurons, some of which have neurites that overlap the dendrites of the cibarial motoneurons and that generate a GRASP-positive signal (Feinberg et al., 2008; Gordon and Scott, 2009) when half of a split-GFP is expressed in the interneuron and the other half in the cibarial motoneurons.
Foraging strategies
The environment and evolution act to generate a vast diversity of foraging strategies in insects. The postdoctoral visiting scientist Juliana Cordeiro from Christen Mirth’s lab will perform a screen to identify elements of the circuit required for the regulation of larval foraging strategies.
The Mirth lab has surveyed foraging behavior in 47 species of Drosophila. When placed on food larvae either eat on the surface of the food, or they burrow into the food, or they disperse and crawl away searching for new food source. Mirth and co-workers have found several closely related species that differ in their burrowing rates and in their burrowing distributions on the food (Mirth, Riddiford, unpublished). D. ananassae, when placed on a food substrate, burrows into the food at high rates. Closely related species, D. bipectinata, D. malerkotliana, D. pallidosa and D. parabipectinata show dramatically different responses; when placed on food, larvae of these species show an extended search phase often crawling straight off the substrate. Larvae of different species also differ in a wide range of responses to social contact that occur on food.
To characterize the populations of cells responsible for burrowing in food and for extended search behavior on food, Cordeiro and Mirth will characterize burrowing and extended search behaviors in wild type D. melanogaster. For each line, they will complete ten 45-minute runs of 100-120 larvae on 245 mm plates containing sucrose-yeast media. They will use the Multi Worm Tracker to monitor larval behavior and the Zlatic lab pipeline to automatically score the number of larvae on the surface of the food and digging events. Next, they will conduct a behavioral screen by selectively activating subsets of larval neurons using the 1200 sparsely expressed GAL4 lines and trpA1. Any lines in which less than 50% of the larvae burrow or that result in larvae that burrow twice as quickly will be retested using shibirets. The data generated during these assays will indicate which groups of neurons regulate burrowing and extended search behavior. In future studies, the Mirth lab will examine how genetic differences between species alter the activity of this circuit to produce species-specific behaviors.
Secondary Assays
Following the identification of neurons required for reacting to sensory stimuli, higher order neurons that were found not to be required in the primary behaviors will be tested for their involvement in learning associations between different sensory modalities and other more complex behaviors.
For this purpose the same hardware modules for automated presentation of stimuli that were used in the primary screens will also be used for the secondary screens. In some cases two different modules will be combined on the same rig. Likewise, the same Multi Worm Tracker software and the same pipeline for data analysis will be used, as described.
Light-odor and light-shocks associations
After completing the primary phototaxis screens, the postdoctoral visiting scientist Naryttza Diaz from Simon Sprecher’s lab will perform a secondary screen to identify neurons required for larval ability to associate light or dark with punishment.
In addition to being able to navigate light gradients, larvae are also able to form associations between light and dark and appetitive or aversive stimuli (Gerber et al., 2004). The neurons harboring the memory trace have not been identified and the mechanisms by which the visual circuit forms associative memories are not known.
Light stimulation can be paired with aversive or attractive gustatory or olfactory stimuli in a classical Pavlovian conditioning paradigm. Automation of the learning paradigms has been technically challenging and generally results in lower performance scores. The Sprecher lab established a stable paradigm for automated olfactory (aversive/appetitive) and electro-shock paradigms.
For this screen the hardware modules for automated presentation of light (used in the phototaxis screen) will be combined on the same rig with the stimulus module for automated presentation of odors (used in the primary chemotaxis screen) or with a module for electric shock presentation developed in the Sprecher lab.
This rig will be used for training larvae to associate light or dark with absence or presence of odors or electric shock. Following several training cycles, larvae will be presented with a choice of light or dark areas and larval choice will be monitored using the Multi Worm Tracker, and the data will automatically be analyzed by the Zlatic lab pipeline.
Odor-food associations
The Gerber lab has pioneered the use of larval Drosophila as a robust model system for associative learning research (Gerber et al., 2004; Gerber and Stocker, 2007; Scherer et al., 2003). A visiting scientist from Bertram Gerber’s lab, together with a visiting scientist from Andreas Thum’s lab will use odor-food associative learning in larva to identify neurons that may be involved in mediating specific interactions between the olfactory pathway, modulatory neurons and neuronal output during odor-food associative learning.
Using the numerically simple larval olfactory pathway, the Gerber lab has provided evidence that embryonic-born intrinsic mushroom body neurons (Kenyon cells) are necessary and sufficient to form appetitive olfactory associations in the larva. This system can provide important insights into how a small set of identified Kenyon cells can store and integrate olfactory information.
The first aim is to identify larval mushroom body neurons involved in the storage and/or retrieval of odor-food memory traces. The second aim is to unravel the connection between these neurons and those of the gustatory processing and motor circuitry pathways. The Gerber lab is interested in understanding how a simple decision, such as “Should a learned response be expressed, or not?” is implemented by neurons. This decision is characterized by the triadic integration of: (1) an activated memory trace; (2) an evaluation of the present situation; and (3) the available behavioral options (Gerber and Hendel, 2006). To this end, this project will capitalize on the work of olfactory, gustatory processing and motor control uncovered in the primary screens.
Anatomically preselected GAL4 lines that express in larval mushroom body extrinsic neurons will be tested for their requirement in storage and/or retrieval of odor-food memory traces. Those neurons required for storage would be candidate mushroom body input neurons. For those neurons required for retrieval, the connection of these neurons with gustatory processing (identified in the primary screens) and motor circuitry (identified in the primary screens) will be anatomically investigated. This should provide an understanding of the triad of memory trace, situational value, and behavioral options, and thus of a prototypically simple 'decision'.
The same stimulus presentation hardware module used in the primary olfactory screen will be used here. During training, odors will be paired either with the presence or absence of food. For testing, two opposing odor gradients will be automatically presented to the animals and larval choice will be monitored with the Multi Worm Tracker, and the data will be automatically analyzed by the Zlatic lab pipeline.
Odor-sound associations
A visiting scientist from the Gerber lab will perform a screen to identify neurons required in forming and retrieving associations between odors and somatosensory stimuli.
The Gerber lab has already successfully used a somatosensory stimulation module developed by the Zlatic lab in a paradigm that uses associations of odor with vibration. Specifically, they trained animals so that one odor is punished, while another odor is not; then, the larva’s choice between the two odors is measured.
For this screen, the Samuel/Louis module for automated odor presentation will be combined with the Zlatic lab module for automated sound presentation on a single rig. This rig will be used for training larvae to associate a specific odor with absence or presence of sound (startling stimulus). Following several training cycles, two opposing odor gradients will be automatically presented to the animals and larval choice will be monitored with the Multi Worm Tracker, and the data will be automatically analyzed by the Zlatic lab pipeline.
The primary screens should identify neurons required for mechanosensation, olfaction, and locomotion. Lines with phenotypes in these assays would not be screened in this association assay; in this way, we can greatly increase the chance that lines with phenotypes will reveal those neurons specifically required for memory formation and/or its retrieval. In turn, the results of the mechanosensation, olfaction, and locomotion screens will enable placement of these neurons into the context of the sensory-motor connectivity.
Learning to prefer odors of their own species (odor imprinting)
Wild type Drosophila melanogaster larvae prefer to spend time in areas containing odors of their own species. Neither anosmic 83b mutant larvae (Fishilevich et al., 2005) nor memory deficient syn97CS mutant larvae (Michels et al., 2005) discriminate between the odors of their own species and those of another species. These data suggest that odor perception and memory are both necessary for the observed behavioral preference, which likely represents odor imprinting (Godoy-Herrera, Medina, Gerber et al., unpublished).
To identify neural substrates that underlie this odor imprinting, a visiting scientist from Raul Godoy-Herrera’s lab will perform a simple high-throughput screen for GAL4/UAS-KiR larvae that lose the capability to prefer odors from their own species. In this screen, a filter paper impregnated with odors from their own species is placed on one side of the tracking arena and a filter paper impregnated with odors from another species is placed on the opposite side. The position of larvae will be monitored by the Multi Worm Tracker and the time that larvae spend on each side of the arena will automatically quantified by the Zlatic lab pipeline.
Preliminary Results

Diagram of a tracking system developed by the Zlatic lab with stimulus modules for automated delivery of air puffs, sound and pain. Figures 1B and 1C show examples of the automated tracking of larvae behavior with Multi Worm Tracker software.

A) Graph shows mean speed (in mm/sec) as a function of time for wild type control larvae. In response to the onset of blue light larvae reduce their crawling speed. Interestingly, in response to the offset of the stimulus, larvae increase their crawling speed. (B-D) Histograms show frequency of peristaltic waves for the wild type larvae before (B) and after the onset (C) and after the offset (D) of the blue light.
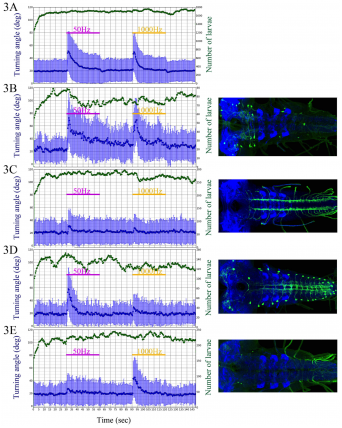
Graphs show mean head turning angle (in degrees) as a function of time before, during and after vibration stimuli for wild type control larvae and 4 examples of “hits” from the inactivation screen. A) Wild-Type. B) Increased activity. C) Decreased activity. D and E) Reduced activity to the 1000 Hz and 500 Hz stimulus respectively.
References
Boyan, G.S., and Ball, E.E. (1993). The grasshopper, Drosophila and neuronal homology (advantages of the insect nervous system for the neuroscientist). Prog Neurobiol 41, 657-682.
Caldwell, J.C., Miller, M.M., Wing, S., Soll, D.R., and Eberl, D.F. (2003). Dynamic analysis of larval locomotion in Drosophila chordotonal organ mutants. Proc Natl Acad Sci USA 100, 16053-16058.
Cheng, L.E., Song, W., Looger, L.L., Jan, L.Y., and Jan, Y.N. (2010). The role of the TRP channel NompC in Drosophila larval and adult locomotion. Neuron 67, 373-380.
Cherbas, L., Hu, X., Zhimulev, I., Belyaeva, E., and Cherbas, P. (2003). EcR isoforms in Drosophila: testing tissue-specific requirements by targeted blockade and rescue. Development 130, 271-284.
Dominick, O.S., and Truman, J.W. (1985). The physiology of wandering behaviour in Manduca sexta. II. The endocrine control of wandering behaviour. J Exp Biol 117, 45-68.
Feinberg, E.H., Vanhoven, M.K., Bendesky, A., Wang, G., Fetter, R.D., Shen, K., and Bargmann, C.I. (2008). GFP Reconstitution Across Synaptic Partners (GRASP) defines cell contacts and synapses in living nervous systems. Neuron 57, 353-363.
Fishilevich, E., Domingos, A.I., Asahina, K., Naef, F., Vosshall, L.B., and Louis, M. (2005). Chemotaxis behavior mediated by single larval olfactory neurons in Drosophila. Curr Biol 15, 2086-2096.
Gerber, B., and Hendel, T. (2006). Outcome expectations drive learned behaviour in larval Drosophila. Proc Biol Sci 273, 2965-2968.
Gerber, B., Scherer, S., Neuser, K., Michels, B., Hendel, T., Stocker, R.F., and Heisenberg, M. (2004). Visual learning in individually assayed Drosophila larvae. J Exp Biol 207, 179-188.
Gerber, B., and Stocker, R.F. (2007). The Drosophila larva as a model for studying chemosensation and chemosensory learning: a review. Chem Senses 32, 65-89.
Gerber, B., Stocker, R.F., Tanimura, T., and Thum, A.S. (2009). Smelling, tasting, learning: Drosophila as a study case. Results Probl Cell Differ 47, 139-185.
Godoy-Herrera, R. (1977). Inter- and intrapopulational variation in digging in Drosophila melanagaster larvae. Behav Genet 7, 433-439.
Gordon, M.D., and Scott, K. (2009). Motor control in a Drosophila taste circuit. Neuron 61, 373-384.
Grillner, S., and Jessell, T.M. (2009). Measured motion: searching for simplicity in spinal locomotor networks. Curr Opin Neurobiol 19, 572-586.
Hughes, C., and Thomas, J. (2007). A sensory feedback circuit coordinates muscle activity in Drosophila. Molecular and Cellular Neuroscience 35, 383-396.
Hwang, R.Y., Zhong, L., Xu, Y., Johnson, T., Zhang, F., Deisseroth, K., and Tracey, W.D. (2007). Nociceptive neurons protect Drosophila larvae from parasitoid wasps. Curr Biol 17, 2105-2116.
Ladle, D., Pechovrieseling, E., and Arber, S. (2007). Assembly of Motor Circuits in the Spinal Cord: Driven to Function by Genetic and Experience-Dependent Mechanisms. Neuron 56, 270-283.
Liu, L., Yermolaieva, O., Johnson, W.A., Abboud, F.M., and Welsh, M.J. (2003). Identification and function of thermosensory neurons in Drosophila larvae. Nat Neurosci 6, 267-273.
Louis, M., Huber, T., Benton, R., Sakmar, T.P., and Vosshall, L.B. (2008a). Bilateral olfactory sensory input enhances chemotaxis behavior. Nat Neurosci 11, 187-199.
Louis, M., Piccinotti, S., and Vosshall, L.B. (2008b). High-resolution measurement of odor-driven behavior in Drosophila larvae. J Vis Exp.
Luo, L., Gershow, M., Rosenzweig, M., Kang, K., Fang-Yen, C., Garrity, P.A., and Samuel, A.D. Navigational decision making in Drosophila thermotaxis. J Neurosci 30, 4261-4272.
Masuda-Nakagawa, L.M., Awasaki, T., Ito, K., and O'Kane, C.J. (2010). Targeting expression to projection neurons that innervate specific mushroom body calyx and antennal lobe glomeruli in larval Drosophila. Gene Expr Patterns 10, 328-337.
Masuda-Nakagawa, L.M., Gendre, N., O'Kane, C.J., and Stocker, R.F. (2009). Localized olfactory representation in mushroom bodies of Drosophila larvae. Proc Natl Acad Sci U S A 106, 10314-10319.
Michels, B., Diegelmann, S., Tanimoto, H., Schwenkert, I., Buchner, E., and Gerber, B. (2005). A role for Synapsin in associative learning: the Drosophila larva as a study case. Learn Mem 12, 224-231.
Murphey, R.K., Possidente, D., Pollack, G., and Merritt, D.J. (1989). Modality-specific axonal projections in the CNS of the flies Phormia and Drosophila. J Comp Neurol 290, 185-200.
Pfeiffer, B.D., Jenett, A., Hammonds, A.S., Ngo, T.T., Misra, S., Murphy, C., Scully, A., Carlson, J.W., Wan, K.H., Laverty, T.R., et al. (2008). Tools for neuroanatomy and neurogenetics in Drosophila. Proc Natl Acad Sci U S A 105, 9715-9720.
Pfeiffer, B.D., Ngo, T.T., Hibbard, K.L., Murphy, C., Jenett, A., Truman, J.W., and Rubin, G.M. Refinement of tools for targeted gene expression in Drosophila. Genetics 186, 735-755.
Sawin-McCormack, E.P., Sokolowski, M.B., and Campos, A.R. (1995). Characterization and genetic analysis of Drosophila melanogaster photobehavior during larval development. J Neurogenet 10, 119-135.
Scherer, S., Stocker, R.F., and Gerber, B. (2003). Olfactory learning in individually assayed Drosophila larvae. Learn Mem 10, 217-225.
Schoofs, A., Niederegger, S., van Ooyen, A., Heinzel, H.G., and Spiess, R. (2010). The brain can eat: establishing the existence of a central pattern generator for feeding in third instar larvae of Drosophila virilis and Drosophila melanogaster. J Insect Physiol 56, 695-705.
Schrader, S., and Merritt, D.J. (2000). Central projections of Drosophila sensory neurons in the transition from embryo to larva. J Comp Neurol 425, 34-44.
Song, W., Onishi, M., Jan, L.Y., and Jan, Y.N. (2007). Peripheral multidendritic sensory neurons are necessary for rhythmic locomotion behavior in Drosophila larvae. Proc Natl Acad Sci USA 104, 5199-5204.
Sprecher, S.G., Pichaud, F., and Desplan, C. (2007). Adult and larval photoreceptors use different mechanisms to specify the same Rhodopsin fates. Genes Dev 21, 2182-2195.
Stocker, R.F. (2009). The olfactory pathway of adult and larval Drosophila: conservation or adaptation to stage-specific needs? Ann N Y Acad Sci 1170, 482-486.
Strausfeld, N. (1976). Atlas of an insect brain (Berlin, Springer-Verlag).
Suster, M.L., and Bate, M. (2002). Embryonic assembly of a central pattern generator without sensory input. Nature 416, 174-178.
Tracey, W.D., Jr., Wilson, R.I., Laurent, G., and Benzer, S. (2003). painless, a Drosophila gene essential for nociception. Cell 113, 261-273.
Xiang, Y., Yuan, Q., Vogt, N., Looger, L.L., Jan, L.Y., and Jan, Y.N. (2010). Light-avoidance-mediating photoreceptors tile the Drosophila larval body wall. Nature 468, 921-926.
Younossi-Hartenstein, A., Salvaterra, P.M., and Hartenstein, V. (2003). Early development of the Drosophila brain: IV. Larval neuropile compartments defined by glial septa. J Comp Neurol 455, 435-450.
