The Light Microscopy Facility offers one-on-one technical support, consultation, and training to ensure optimal use of our many advanced capabilities. Please contact us to help identify the best instrumentation for your imaging needs.
Note that most microscopes are equipped with temperature and CO2 incubation control for live cell, time-lapse imaging. Auto-focusing and other stabilization modules such as Perfect Focusing System (PFS) minimize drift in real time.
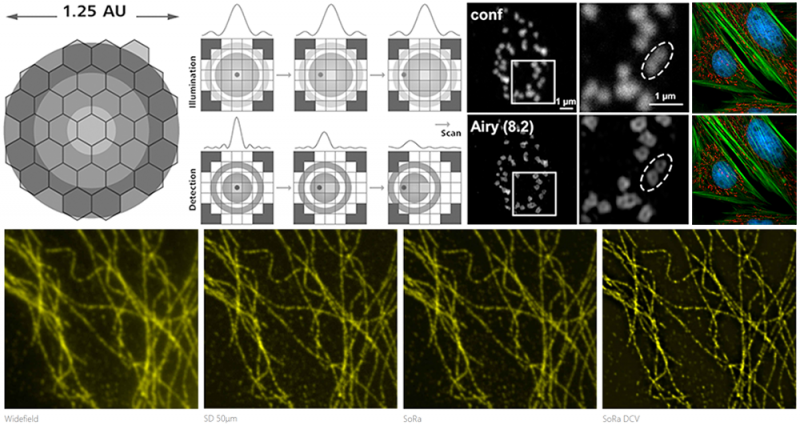
Enhanced Resolution - Pixel Reassignment:
Confocal microscopes use a pinhole to reject out-of-focus light to improve image contrast. They also benefit from a slight increase in resolution since the detection PSF is convolved with the physical pinhole; however, the achievable resolution is often limited. While the probable position of a fluorophore is within the narrow overlap between the illumination and detection PSFs, it is actually detected along the optical axis corresponding to the detector position leading to resolution loss. The goal of pixel reassignment is to shift the measured signal back to where the effective PSF is located. Digital approaches such as Airyscan or optical approaches such as SoRa can provide two-fold resolution enhancement without requiring any specific sample preparation protocol. See Additional Information.
- Zeiss LSM 980 with Airyscan and Zeiss LSM 980 NLO with Airyscan are confocal microscopes designed for optimized productivity using a 32-element Airyscan array detector enabling resolution improvement in all three dimensions and higher sensitivity with gentle illumination, making it ideal for dim samples.
- Nikon CSU-W1 SoRa with TIRF, Nikon CSU-W1 SoRa, and VisiTech iSIM are spinning disk and array-scanning confocal microscopes with microlensed arrays enabling superior spatiotemporal resolution for comparatively larger FOVs, making them suitable for fast, live experiments.
- MOSAIC combines several imaging modalities with camera-based pixel reassignment and adaptive optics.
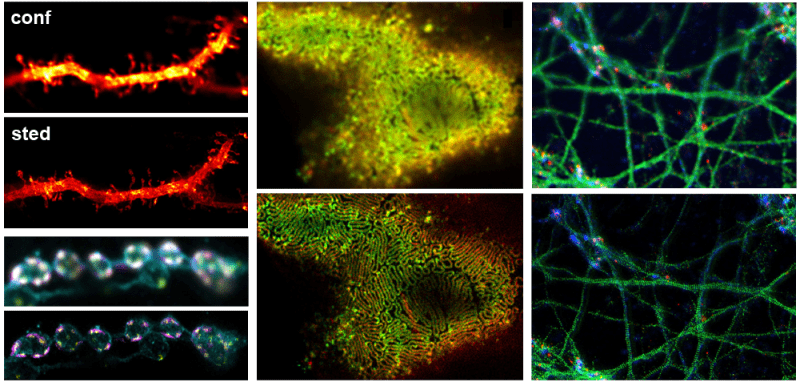
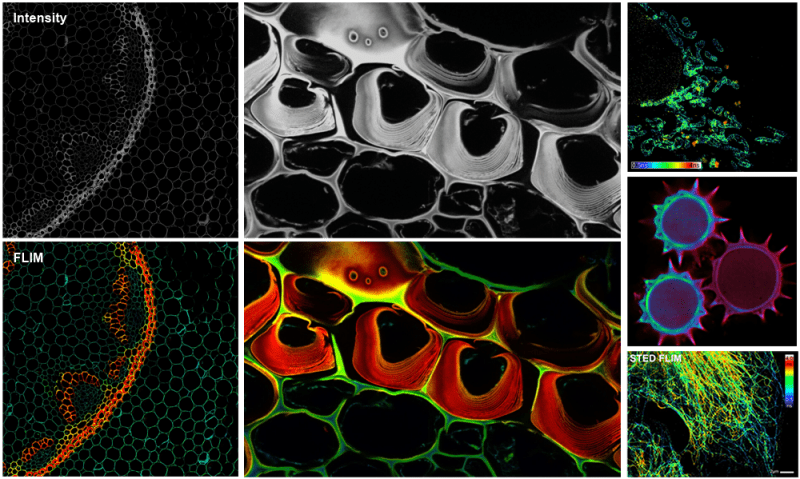
Super Resolution - Stimulated Emission Depletion (STED) Microscopy:
STED is a point scanning technique that combines an excitation and depletion laser to activate a subpopulation of fluorophores at the center of a focal spot while suppressing neighboring fluorescence. The depletion beam is shaped to achieve 2D or 3D confinement with typical lateral resolution as high as 20 nm. Comparatively, STED restricts the types of dyes used during sample preparation; recommended dyes and media can be found online. See Additional Information.
- Abberior Expert Line STED is a turn-key confocal microscope engineered for 2D and 3D STED microscopy with multiple depletion lasers. A full auto-alignment procedure enables immediate imaging. The configuration is highly flexible and optimized for live imaging.
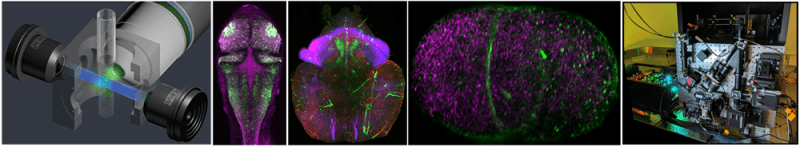
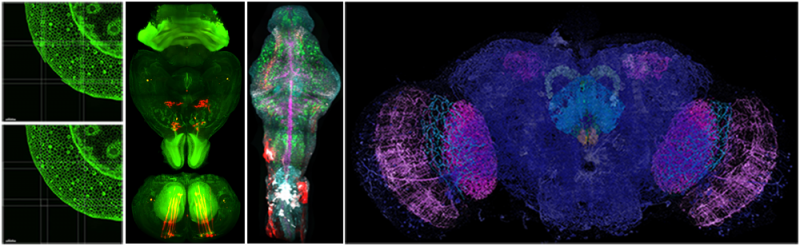
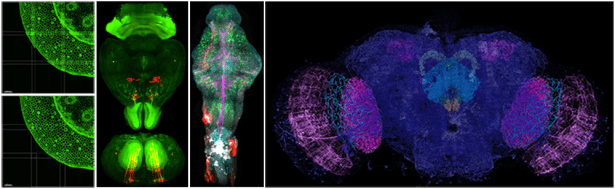
Light Sheet Fluorescence Microscopy (LSFM):
LSFM or Selective Plane Illumination Microscopy (SPIM) allows fast 3D imaging of large volumes. By gently illuminating a thin section (usually a few hundred nanometers to several microns) of the sample at any given time, this method greatly reduces photodamage and stress on a live sample while achieving good optical sectioning capabilities. In contrast to point scanning techniques, LSFM can acquire images at speeds 100-1000 times faster. See Additional Information.
- Zeiss Lightsheet 7 and Luxendo MuVi SPIM are turn-key instruments for routine high-speed 3D imaging with intermediate axial resolution. It is tailored for zebrafish and drosophila embryos, whole mouse brains, expanded samples, and transparent specimens (up to 5 mm).
- Lattice Light Sheet is a custom-built microscope employing an optical lattice of Bessel beams to generate an ultra-thin light sheet resulting in superior axial resolution. It is tailored for zebrafish and drosophila embryos, expanded samples, and transparent specimens (up to 4 mm).
- MOSAIC is a custom-built system combining several imaging modalities including lattice light sheet microscopy with adaptive optics that are vital for visualizing and quantifying complex biological systems with high spatiotemporal resolution.
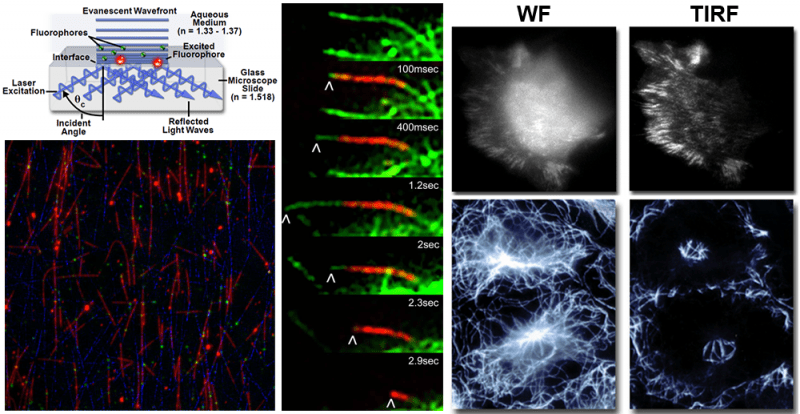
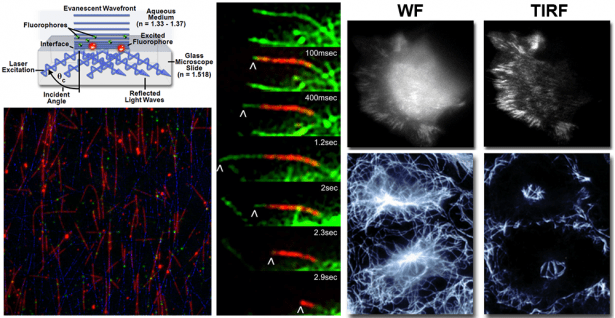
Total Internal Reflection Fluorescence (TIRF) Microscopy:
TIRF is a powerful widefield imaging technique that achieves superior optical sectioning for specimens close to the coverslip. An evanescent wave decays exponentially at the glass-water interface achieving a penetration depth of approximately 100 nm into the sample medium. The elimination of background fluorescence improves SNR and permits true diffraction-limited imaging. See Additional Information.
- Nikon CSU-W1 SoRa with TIRF is an inverted spinning disk confocal / TIRF microscope with a 60x/1.49 oil objective. The motorized TIRF-E module is semi-automated for accurate and consistent alignment.
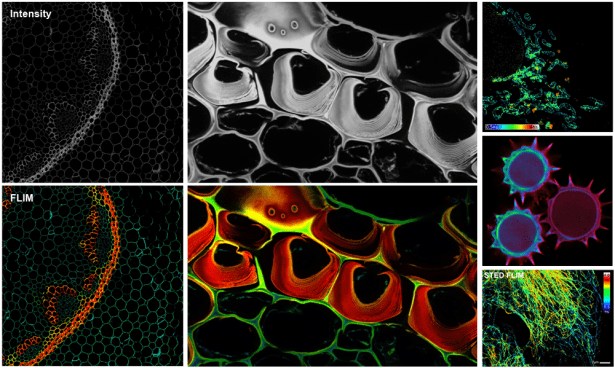
Fluorescence Lifetime Imaging Microscopy (FLIM):
Since contrast is based on the lifetime of individual fluorophores rather than their emission spectra, FLIM imaging can be more robust than intensity based methods. In lieu of spectral imaging which involves linear unmixing post-acquisition, FLIM can differentiate spectrally-overlapping and/or weakly-fluorescent dyes, including cellular autofluorescence, in real time. See Additional Information.
- Abberior Expert Line STED is a confocal microscope equipped with 4 spectral APDs. The configuration is highly flexible, optimized for live imaging, as well as being compatible with STED and the MATRIX detector for improved SNR and resolution.
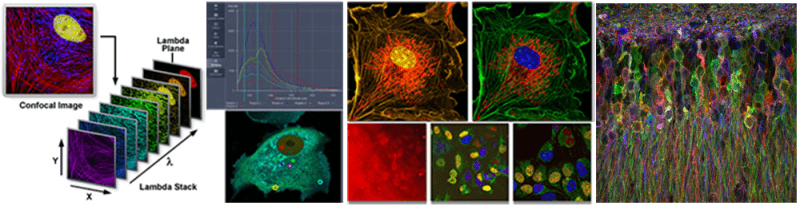
Spectral Imaging (linear unmixing):
Spectral imaging combined with linear unmixing is a highly useful technique that can be used in combination with other advanced imaging modalities to untangle fluorescence spectral overlap artifacts in cells and tissues labeled with synthetic fluorophores that would be otherwise difficult to separate. The spectral detector (lambda mode) is used to measure fluorescence emission over a broad range of wavelengths. Using reference emission spectra ('fingerprints'), linear unmixing determines the relative contribution of each fluorophore in every pixel of an image. Consequently, separation of up to eight spectrally overlapping dyes as well as uncharacterized autofluorescence is possible while potentially reducing imaging time by acquiring all dyes in parallel and unmixing them post-acquisition.
- Zeiss LSM 980 with Airyscan and Zeiss LSM 980 NLO with Airyscan are confocal microscopes with a 32-element internal detector. A 'lambda stack' is processed using the Linear Unmixing tool in the Zen software such that reference spectra are obtained interactively or automatically using "Multi-channel Unmixing" or "Automatic Component Extraction".
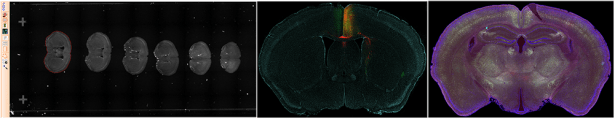
High-throughput Imaging (automated acquisitions):
High-throughput imaging is the use of automation equipment using classical techniques from optics, chemistry, biology, or image analysis to permit rapid, highly parallel research. One of the most common technologies employed in microscopy is slide scanning which allows hundreds of slides (e.g., sectioned mouse brain) to be imaged in a single run using a shared set of acquisition settings.
- TissueGnostics TissueFAXS 200 is a combination widefield and spinning disk confocal microscope operated as a whole slide digital scanner; optical sectioning from the latter imaging modality offers increased contrast based on rejection of out-of-focus light. Faster acquisitions are possible by only imaging the tissue sections ("automated" detection).
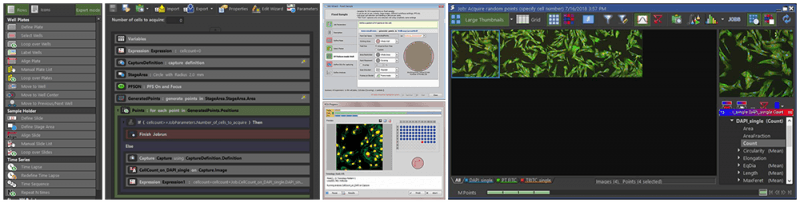
High Content Imaging (automated acquisitions):
Custom acquisitions of sample carriers and multi-well plates can be automated without the need for advanced macros or scripting. Based on real-time image analysis and feedback, acquisitions settings can be programmatically modified on-the-fly.
- Nikon CSU-W1 SoRa with TIRF, Nikon CSU-W1 SoRa, and Nikon Eclipse Ti can accommodate a range of sample carriers and multi-well plates. Using NIS-Elements, the JOBS module offers programmatic control and python scripting tailored for advanced acquisitions while also making use of High Content Analysis software with heat maps representation to automatically quantify data on-the-fly. Similarly, VisiTech iSIM is controlled by VisiView software for advanced control with python scripting.
Advanced Image Processing - Deconvolution:
Deconvolution is an image processing technique used to improve the contrast and resolution of images captured using an optical microscope. Out-of-focus light causes blur in a digital image. Mathematically, this can be represented as a convolution operation. Deconvolution seeks to remove or reassign this light present in 2D and 3D image acquisitions in order to actually attain the desired optical resolution of the imaging system. Nearly all fluoresence images can and should be deconvolved, with advanced techniques like confocal and super resolution microscopy also realizing its benefits. Note that if a system is already diffraction-limited then deconvolution, alone, will not improve resolution nor if sampling is greater than the Nyquist limit. For more information, please see Supplementary Microscope Objectives.
- Zen is the control and processing software for Zeiss microscopes. Data acquired on these microscopes can be deconvolved using an estimated or experimentally-measured PSF. All imaging modalities, especially Airyscan, are supported.
- NIS Elements is the control and processing software for Nikon microscopes. Data acquired on these microscopes can be deconvolved using an estimated or experimentally-measured PSF. All imaging modalities, especially SoRa, are supported.
- Imaris is visualization and analysis software capable of interactive 3D/4D rendering for large, multi-channel datasets (for most image formats), acquired with any microscope. Light sheet templates and the ability to use an experimentally-measured PSF are lacking.
- FIJI is an extended distribution of ImageJ with many bundled 3rd party plugins, such as DeconvolutionLab2. As an open-source alternative, it is robust with multiple algorithms available although it is computationally intensive.
- Image Processing Pipeline (IPP) offers advanced workflows for light sheet acquisitions. Single volume or tiled acquisitions can be deconvolved quickly on Janelia's compute cluster using an experimentally-measured PSF.
Advanced Image Processing - Big Data Stitching/Fusion:
For tiled acquisitions on light sheet microscopes, it is necessary to align and fuse all tiles/views. The size of typical datasets can range up to several TB; consequently, only certain applications are capable of properly handling 'big data' in an accurate and timely manner.
- Imaris Stitcher is a stand-alone application for aligning and fusing microscopy image tiles. Individual z-stacks will be shifted with respect to one another to ensure best results; however, the amount of overlap between tiles and the available computer memory will ultimately determine alignment precision. It is ideal for mouse brain and low-resolution acquisitions.
- FIJI is an extended distribution of ImageJ with many bundled 3rd party plugins, such as BigStitcher. As an open-source alternative, it offers simple and efficient alignment of multi-tile and multi-angle image datasets although it is computationally intensive.
- IPP offers advanced workflows for light sheet acquisitions by providing a web interface to easily process (on Janelia's compute cluster) and export data in a user-friendly format. Only those modules desired need to be selected including:
- Flatfield Correction: This procedure compensates for illumination inhomogeneity in the FOV (intensity drop off at edges); a reference image is back calculated from the data itself, thus acquiring more images should yield better results.
- Deconvolution: It may be necessary to perform deconvolution (deblurring) to achieve the resolution capable for the microscope in addition to improved contrast. A Richardson-Lucy iterative scheme is used with an experimentally-measured PSF such that the number of iterations (strength) can be specified.
- Stitching: The algorithm applies affine transformations that also grows/shrinks neighboring tiles for better alignment. The size of the dataset should not limit performance.